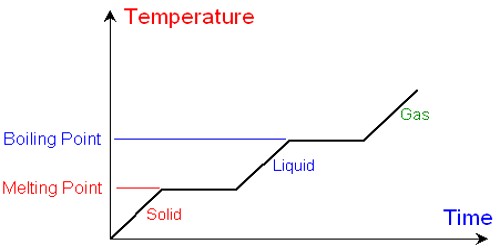
The melting point of a substance is the temperature at which this substance goes from the solid-state to the liquid one, at normal pressure. It is the temperature at which a solid substance melts or fuses. For water, this is 0° Celsius (32 Fahrenheit, 273,15 Kelvin). The chemical element with the highest melting point is tungsten. The transition between the solid and the liquid is so sharp for small samples of a pure substance that melting points can be measured to 0.1oC. The melting point of solid oxygen, for example, is -218.4oC. Some chemical compounds have a higher melting point.
The melting point depends on pressure, so it should be specified. When looking at when a liquid substance becomes solid, most people call this the freezing point. Under standard atmospheric pressure different pure crystalline solids will each melt at different specific temperature; thus melting point is a characteristic of a substance and can be used to identify it. For most substances, like water, this is the same as the melting point. Typically, tables of melting points are for standard pressure, such as 100 kPa or 1 atmosphere. There are substances, where this is not the case (they melt at one temperature, and freeze at another). Agar seems to melt at 85° Celsius but freeze at between 35°C and 40°C. This phenomenon is a kind of hysteresis. A pure substance under a standard condition of pressure has a single reproducible melting point.
Adding certain substances or impurities will change the melting point of the resulting mixture. For example, sugar or salt will lower the melting point, and alcohol will raise it. he terms melting point and freezing point are often used interchangeably, depending on whether the substance is being heated or cooled.