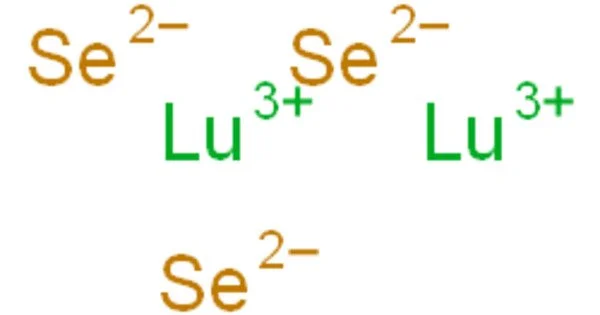Lutetium selenide is an inorganic compound with the chemical formula Lu2Se3. It is a compound composed of lutetium (Lu) and selenium (Se). It can be obtained by reacting lutetium and selenium or lutetium oxide and hydrogen selenide at a high temperature. It can form orthorhombic AgLuSe2 in the binary system of silver selenide. It can form Lu2PbSe4 and Lu2Pb4Se7 in the binary system of lead selenide.
Lutetium is a rare earth element belonging to the lanthanide series of the periodic table, while selenium is a non-metal in the chalcogen group. Lutetium selenide is part of a family of materials known as metal selenides, where a metal element forms compounds with selenium.
Properties
Lutetium(III) selenide can form orthorhombic AgLuSe2 in the binary system of silver selenide. It can form Lu2PbSe4 and Lu2Pb4Se7 in the binary system of lead selenide.
- Chemical formula: Lu2Se3
- Molar mass: 586.847 g·mol−1
- Appearance: grey
Preparation
Lutetium(III) selenide can be obtained by reacting lutetium and selenium:
2 Lu + 3 Se → Lu2Se3
It can also be prepared by reacting lutetium oxide and hydrogen selenide at a high temperature:
Lu2O3 + 3 H2Se → Lu2Se3 + 3 H2O
These compounds often have interesting electronic, optical, and magnetic properties, making them of interest for various applications in materials science and technology. The specific properties of lutetium selenide would depend on its crystal structure, which can vary based on factors like temperature and pressure.