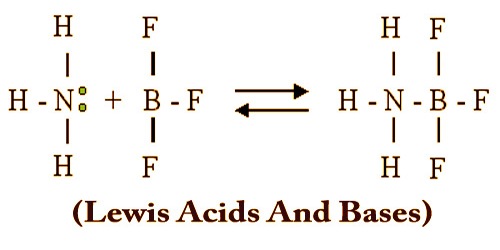Lewis acids and bases are named after the American chemist Gilbert Newton Lewis, who has also made invaluable contributions in the thermodynamics and photochemical fields. A Lewis acid is a chemical substance that comprises an empty orbital capable of accepting a Lewis dependent electron pair to form a Lewis adduct. Among other words, a Lewis acid is an acceptor of electron pairs. A Lewis base, then, is any species that contains a filled orbital containing an electron pair which isn’t involved in bonding but may form a covalent bond with a Lewis acid to create a Lewis adduct.
Bases donate pairs of electrons in Lewis theory of acid-base reactions, and acids accept pairs of electrons. An example of such a Lewis acid would be BR3 (where R can be a halide or an organic substituent). Water and certain other compounds are called Lewis acids as well as bases since they can accept and give pairs of electrons depending on the reaction. The terms nucleophile and electrophile, respectively, are more or less synonymous with Lewis base and Lewis acid.
The Lewis theory’s one advantage is the way it complements the oxidation-reduction reaction model. Oxidation-reduction reactions require the movement of electrons from one atom to another, with a net change in one or more atoms’ oxidation numbers. In many cases, the interaction between the Lewis base and Lewis acid during a complex is indicated by an arrow indicating the Lewis base donating electrons toward the Lewis acid using the notation of a coordinate bond, for example, Me3B←NH3.
Some sources indicate the Lewis base with a pair of dots (the specific electrons being donated), allowing accurate representation of the transition from the base itself to the acid complex:
Me3B + :NH3 → Me3B:NH3
Atomic or molecular chemical molecules with a strongly concentrated HOMO (Highest Occupied Molecular Orbital) serve as bases for Lewis. These chemical species have the flexibility to donate an electron pair to a given Lewis acid so as to make an adduct, as discussed earlier. Although there are attempts to use computational and experimental energetic criteria to differentiate dative bonding from non-dative covalent bonds, for the foremost part, the excellence merely makes note of the source of the electron pair, and dative bonds, once formed, behave simply as other covalent bonds do, though they typically have considerable polar character.
The Lewis theory implies acids combine with bases to share a pair of electrons, with no change in any atom’s oxidation numbers. Many chemical reactions can be divided into one of those groups or another. Either electron are transferred from one atom to a different, or the atoms close to share a pair of electrons. The foremost common Lewis bases are ammonia, alkyl amines, and other conventional amines. Lewis bases are usually anionic in nature, and their base strength depends generally on the pKa of the corresponding parent acid.
In addition, in some situations (e.g., sulfoxides and amine oxides as R2S→O and R3N→O), the use of the dative bond arrow is merely a notational convenience to prevent formal charges being drawn. The biggest advantage of the Lewis principle is the way the number of acids and thus the number of acid-base reactions increases. In the Lewis theory, an acid is an ion or molecule that may accept a pair of nonbonding valence electrons. Since Lewis bases are electron-rich species that have the power to donate electron-pairs, they will be classified as nucleophiles. Likewise, Lewis acids can be classified as electrophiles; because they serve as acceptors of electron pairs.
In the Friedel-Crafts alkylation reaction, a typical example of a Lewis acid in action is. The key step is the acceptance by AlCl3 of a chloride ion lone-pair, forming AlCl4− and creating the strongly acidic, that is, electrophilic, carbonium ion. The theory of Lewis acid-base can also be used to describe why non-metal oxides like CO2 dissolve in water to form acids like carbonic acid H2CO3. Lewis bases have immense applications within the modification of the selectivity and therefore the activity of metallic catalysts. For the assembly of pharmaceuticals, asymmetric catalysis is a crucial part of enantioselective synthesis. Chiral Lewis bases are often used to confer chirality on catalysts, in order to allow asymmetric catalysis.
Information Sources: