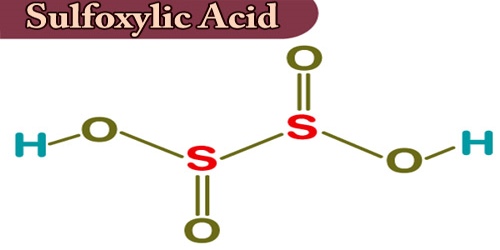
Sulfoxylic acid (H2SO2) is an unstable sulfur oxoacid at an intermediate state of oxidation between hydrogen sulfide and dithionic acid. It is also known as hyposulfuric acid or dihydroxide to sulfur. The molecule containing sulfoxylic acid contains a total of 4 bonds, 2 non-H bonds, and 2 hydroxyl groups. Sulfur monoxide (SO) can be considered for sulfoxylic acid as a potential anhydride but it is not currently known to react with water.
Sulfoxyl acid is a sulfinic acid isomer that has no separate occurrence and is not observed in the aqueous state. The complementary basis for this is the much more stable sulfoxylate anion SO2−2. In between these states is the HSO−2 ion, also somewhat stable. It is assumed that the carbon atoms in the chemical structure of sulfoxylic acid are located at the corner(s), and that hydrogen atoms attached to carbon atoms are not suggested that each carbon atom is known to be associated with sufficient hydrogen atoms to provide four bonds to the carbon atom.
Sulfoxylic acid has been distinguished in the gas stage. It is probably going to be framed as a transitional when hydrogen sulfide is oxidized by living beings, or in the air, or anyplace else in the common habitat. The chemical formula of sulfoxylic acid (H2O2S) is predicated on the formula indicating the numbers of every kind of atom during a molecule without structural information, which is different from the chemical formula which provides the numerical proportions of atoms of every type. When H2S is oxidized it starts from oxidation state −2, and should then pass through intermediate values of 0 and +2 before getting to well-known sulfite at +4 and sulfate at +6.
At the point when sulfide in basic conditions is oxidized via air within the sight of nickel particles, sulfoxylate focus first increments to around 5% and afterward diminishes more than a few days. The synthetic recipe is the premise of stoichiometry in compound conditions, i.e., the figuring of relative amounts of reactants and items in substance responses. The law of protection of mass directs that the amount of every component given in the substance equation doesn’t change in a synthetic response.
Ultraviolet irradiation of a mixture of solid H2S and H2O has created sulfoxylic acid, accompanied by warming. This is a potential mechanism common to comets or circumstellar disks. Thus, based on the chemical formula, each side of the chemical equation must represent the same quantity of any particular element. Sulfoxylic acid is an isomer of sulfinic acid, which has a hydrogen atom bonded to the sulfur, and the oxygen connected with a double bond (HS(O)OH).
Other isomers include thiadioxyrane (a ring of two atoms of oxygen and sulfur), dihydrogen sulfone (a sulfur atom bound to two atoms of hydrogen and two of oxygen), sulfhydryl hydroperoxide (HSOOH), and H2SOO dihydrogen persulfoxide. The lowest energy of all of these isomers is the sulfoxylic acid. Forms hydrogen sulfite and sulphates when sulfoxylate reacts with hypochlorite, bromine, or chlorine dioxide.
Information Sources: