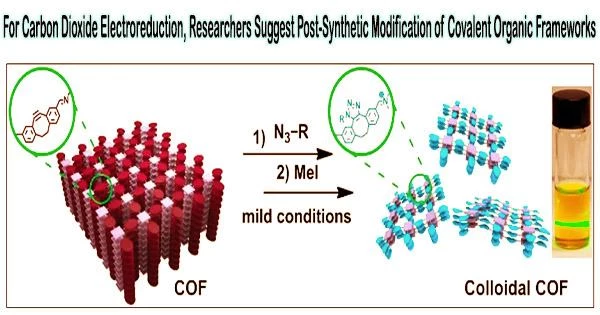
An appropriate class of templates for the construction of electrocatalytic carbon dioxide reduction reaction (CO2RR) catalysts is thought to be covalent organic frameworks (COFs), which have organized pores and high-precision functionalization.
In addition to promoting charge transfer and improving CO2 adsorption, C-N bonds and ionic skeletons can also increase conductivity. However, because of electrostatic repulsion and the weak connection, direct bottom-up synthesis can scarcely achieve the coexistence of C-N bonds and ionic frameworks.
A research team led by Prof. Zeng Gaofeng and Assoc. Prof. Xu Qing from the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences has proposed a multilevel post-synthetic modification strategy to construct catalytic COFs towards CO2RR with high activity and selectivity.
The results were published in Nature Communications.
Catalytic COFs synthesized by the post modification showed a maximum turnover frequency value of 9922.68 h–1 at –1.0 V and the highest faradaic efficiency of 97.32% at –0.8 V, which were higher than that of the base COF and the single-modified COFs.
Ionic skeleton was found to contribute to greater activity, while electrocatalysis studies and characterizations showed that C-N bonds could improve catalytic selectivity.
Additionally, theoretical calculations showed that the rate-determined phase was the simpler instantaneous *CO from COOH* synthesis, and methyl groups strengthened electron density.
This research deepens our understanding of the role of COFs in CO2 reduction reactions. It provides information on building multilevel COFs after synthetic alteration for tailored activity and great stability.