A new study not only sheds light on how the APOE4 gene may cause some of the pathologies associated with Alzheimer’s disease but also suggests a new treatment target that may help people with the APOE4 gene in the early and late stages of the disease. APOE4 has been linked to the activation of an inflammatory protein that causes a breakdown in the blood-brain barrier, which protects the brain.
A new study published in Nature Aging not only sheds light on how the gene may trigger a cascade of pathologies that contribute to Alzheimer’s disease, but also suggests a new treatment target that may help people who carry the APOE4 gene in the early and late stages of the disease. Researchers at USC’s Keck School of Medicine discovered that APOE4 is linked to the activation of an inflammatory protein that causes a breakdown in the blood-brain barrier, which protects the brain.
Like amyloid plaque, the genetic variant APOE4 has long been associated with Alzheimer’s disease, but still little is known about the role the gene plays in the disease process.
This study builds on a recent USC study that found APOE4 causes leaks in the blood-brain barrier in humans, allowing toxic substances from the bloodstream to enter the brain, damaging brain cells, and interfering with cognitive functions. This process causes memory problems in patients regardless of whether their brain shows signs of amyloid-, the sticky plaque peptide thought to be a hallmark of the disease.
The latest findings also point to a new potential treatment to slow or prevent the cognitive decline associated with Alzheimer’s disease in patients who carry the APOE4 gene, regardless of amyloid pathology.
“We’re also focusing on therapeutic targets in blood vessels that could lead to new treatments for people with Alzheimer’s disease, both in the early and late stages of the disease. Current findings in mouse models may be especially promising for treating late-stage disease with advanced amyloid-pathology “Berislav Zlokovic, MD, Ph.D., director of the Zilkha Neurogenetic Institute at USC’s Keck School of Medicine, made the announcement.
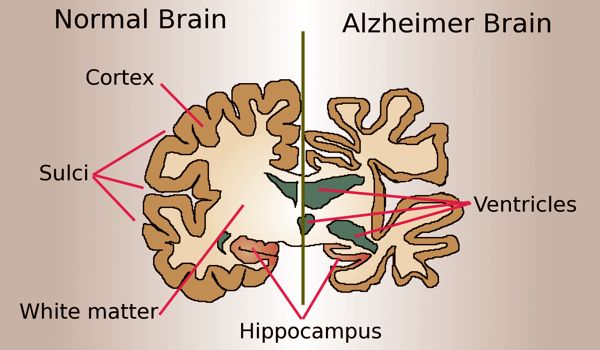
The role of APOE4, pericytes and Cyclophilin A in Alzheimer’s disease
APOE4 has been shown to hasten the breakdown of the blood-brain barrier by damaging pericytes, a layer of cells that strengthens and protects the brain capillaries that comprise the blood-brain barrier. This breakdown is also linked to higher levels of Cyclophilin A, a pro-inflammatory protein, in the brain vessels of APOE4-positive Alzheimer’s disease patients.
USC researchers focused on Cyclophilin A in mice with the APOE4 gene, which carries a high risk of Alzheimer’s disease, and mice with the APOE3 gene, which carries an average risk of Alzheimer’s disease, in this study. Cyclophilin A is found in pericytes and regulates the strength of blood vessels in maintaining the blood-brain barrier’s integrity. Researchers discovered that Cyclophilin A activated matrix metalloproteinase 9 (MMP9), an enzyme that degrades blood vessels in the blood-brain barrier, in APOE4 mice. This did not occur in APOE3 gene mice.
The researchers then tried treating APOE4 mice with a Cyclophilin A inhibitor. The inhibitor not only improved blood-brain barrier integrity in APOE4 mice but also prevented further neuron loss and behavioral deficits. The APOE4 mice treated with the inhibitor did not exhibit behavioral deficits during daily activities, according to the researchers. This suggests that therapies targeting this pathway may be able to slow the progression of vascular and neurodegenerative disorders in people with Alzheimer’s disease who have the APOE4 gene.
“Until now, there has been little hope for those in the late stages of the disease, which is very difficult on patients and their loved ones,” Zlokovic said. “We are excited to investigate the potential for interventions focusing on blood-brain barrier repair and blood vessel strength, independent of amyloid pathology, to slow or stop neurodegeneration and cognitive decline in advanced Alzheimer’s disease.”
The Cyclophilin A inhibitor used in this study, Debio-025, has been used in humans to treat hepatitis C, suggesting that it could be a potential treatment for cognitive impairment in APOE4 carriers who show Cyclophilin A-MMP9 pathway activity in early or late disease stages.