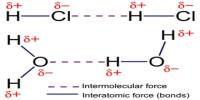
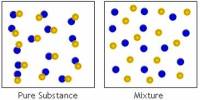
Basic purpose of this lecture is to describe on Atoms and Elements. Here focus on the Atomic Number and Mass Number Isotopes. A particular atom will have the same number of protons and electrons and most atoms have at least as many neutrons as protons. Finally describe on atomic Theory: Atoms are building blocks of elements Similar atoms in each element and Two or more different atoms bond in simple ratios to form compounds.
More Posts
Latest Post
-

Oxprenoate Potassium
-

Crude Oil could be Fractionated with a Revolutionary Method that uses significantly less Energy
-

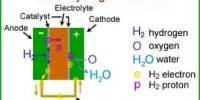
Electric Aircraft may be made possible via a New Fuel Cell
-

Monopotassium Phosphite
-

Researchers Recently made significant Progress on the Quantum Internet
-

Findings could Improve the Performance of Solid-state Batteries