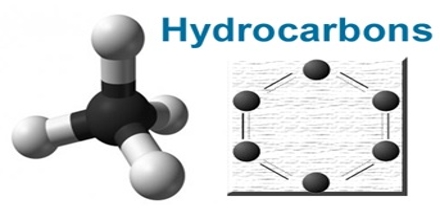
A hydrocarbon is a compound consisting of only hydrogen and carbon. Prime objective of this lecture is to present on Hydrocarbons. The carbon to carbon can be single, double, or triple bonds. The bonds are always nonpolar. Carbon-to-carbon bonds can be single (A), double (B), or triple (C). Note that in each example, each carbon atom has four dashes, which represent four bonding pairs of electrons, satisfying the octet rule. Here also present on Alkane. Alkanes are hydrocarbons with only single bonds. Alkanes occur in what is called a homologous series.
Lecture on Hydrocarbons