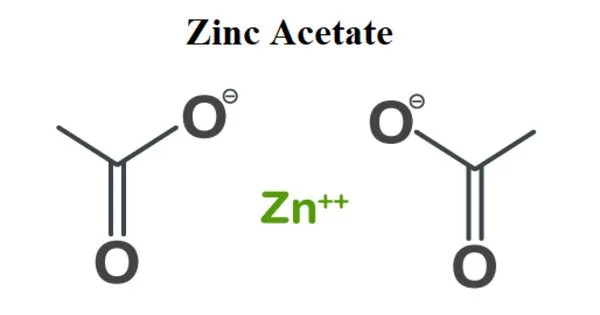
Zinc acetate is an acetate salt in which the cationic component is zinc(2+). It is a salt with the formula Zn(CH3CO)2, which is most commonly found as the dihydrate Zn(CH3CO2)2•2H2O. It serves as an astringent. Both the hydrate and anhydrous forms are colorless solids used as dietary supplements.
Acetic acid reacts with zinc carbonate or zinc metal to produce zinc acetates. It is a zinc molecular entity and an acetate salt. It is designated as E650 when used as a food additive. It is made by reacting zinc oxide (ZnO) with acetic acid. It is widely used as an emetic, astringent, and styptic.
Properties
- Chemical formula: Zn(CH3COO)2(H2O)2 (dihydrate)
- Molar mass: 219.50 g/mol (dihydrate); 183.48 g/mol (anhydrous)
- Appearance: White solid (all forms)
- Density: 1.735 g/cm3 (dihydrate)
- Melting point: Decomposes at 237 °C (459 °F; 510 K) (dihydrate loses water at 100 °C)
- Boiling point: decomposes
- Solubility in water: 43 g/100 mL (20 °C, dihydrate)
- Solubility: 1.5 g/100 mL (methanol)

Structures
In anhydrous zinc acetate the zinc is coordinated to four oxygen atoms to give a tetrahedral environment, these tetrahedral polyhedra are then interconnected by acetate ligands to give a range of polymeric structures.
In the dihydrate, zinc is octahedral, wherein both acetate groups are bidentate.
Production
Anhydrous zinc acetate is produced by reacting zinc nitrate (Zn(NO₃)₂) and acetic anhydride.
Action of acetic acid (CH3COOH) on zinc oxide (ZnO).
Uses
Zinc acetate is found in a variety of medications, including cold lozenges. Zinc acetate is also available as a dietary supplement. As part of the treatment for Wilson’s disease, it is taken orally every day to inhibit the body’s absorption of copper.
For the topical treatment of acne, zinc acetate is also available as an astringent in the form of an ointment, a topical lotion, or in combination with an antibiotic such as erythromycin. It is most commonly available as a topical anti-itch ointment.
Zinc acetate is used as a catalyst in the industrial production of vinyl acetate from acetylene: approximately one-third of global production follows this route, which, due to its environmental ramifications, is mostly practiced in countries with lax environmental regulations, such as China.
Health hazards
Inhaling zinc diacetate may cause mild throat and nose irritation, resulting in sneezing and coughing. Swallowing corrodes or irritates the gastrointestinal tract, resulting in vomiting. It is a non-flammable substance.