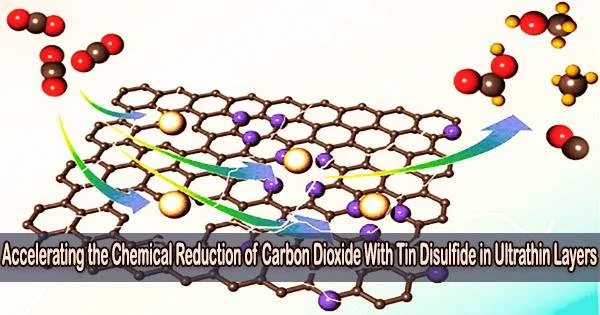
Ultrathin layers of tin disulfide, also known as SnS2, refer to extremely thin films or sheets of the compound tin(IV) sulfide. Tin disulfide is a two-dimensional material that belongs to the family of transition metal dichalcogenides (TMDs). These materials have gained significant attention in the field of nanotechnology and materials science due to their unique electronic, optical, and mechanical properties.
In ACS Nano, researchers from Kanazawa University describe how ultrathin layers of tin disulfide can be utilized to speed up the chemical reduction of carbon dioxide, a discovery that is very important for our effort to create a society that is carbon neutral.
Recycling carbon dioxide (CO2) produced by industrial operations is essential to the urgent goal of a society that is carbon-neutral and sustainable. Electrocatalysts that effectively transform CO2 into different, less harmful chemical compounds are currently being extensively explored for this purpose.
Two-dimensional (2D) metal dichalcogenides are a class of materials that are potential electrocatalysts for CO2 conversion, but because they frequently promote opposing reactions, their effectiveness is diminished.
Yasufumi Takahashi from Nano Life Science Institute (WPI-NanoLSI), Kanazawa University and colleagues have now identified a 2D metal dichalcogenide that can efficiently reduce CO2 to formic acid, a compound that not only occurs naturally but also is an intermediate product in chemical synthesis.
These findings will provide a better understanding and design strategies for metal dichalcogenide-based 2D electrocatalysis for electrochemical CO2 reduction to produce hydrocarbons, alcohols, fatty acids and olefins without by-products.
Yasufumi Takahashi and colleagues
Takahashi and colleagues compared the catalytic performance of 2D sheets of disulfide (MoS2) and tin disulfide (SnS2). Both are 2D metal dichalcogenides, with the latter of relevance due to the fact that pure tin is a well-known catalyst for formic acid synthesis.
Electrochemical tests of these compounds revealed that with MoS2, instead of CO2 conversion, hydrogen evolution reaction (HER) was promoted. When the creation of hydrogen gas fuel is the goal, HER refers to a reaction that produces hydrogen. However, when CO2 reduction is the goal, HER is an unwelcome competing process.
Researchers and scientists are actively studying ultrathin layers of tin disulfide and other two-dimensional materials to understand their fundamental properties and explore their potential in various technological applications, including nanoelectronics, nanophotonics, and energy storage devices.
SnS2, on the other hand, showed good CO2 reduction activity and suppressed HER. The researchers also measured the electrochemical activity of bulk SnS2 powder, which had reduced catalytic CO2 reduction activity.
The researchers used a technique known as scanning electrochemical cell microscopy (SECCM) to determine where the catalytically active spots are in SnS2 and why the 2D material performs better than the bulk component.
To create a meniscus-shaped nanoscale electrochemical cell for the surface reactivity sensing probe on the material, the SECCM is employed as a nanopipette. The results showed that the SnS2 sheet’s entire surface, not just its “terrace” or “edge” features, is catalytically active. This also explains why 2D SnS2 has enhanced activity compared to bulk SnS2.
Calculations provided further insights into the chemical reactions at play. With regard to using 2D SnS2 as a catalyst, the production of formic acid was validated as an energetically advantageous reaction pathway.
The results of Takahashi and colleagues signify an important step forward towards the use of 2D electrocatalysts in electrochemical CO2 reduction applications. They state, “These findings will provide a better understanding and design strategies for metal dichalcogenide-based 2D electrocatalysis for electrochemical CO2 reduction to produce hydrocarbons, alcohols, fatty acids and olefins without by-products.”
Background: Two-dimensional metal dichalcogenides
Two-dimensional (2D) metal dichalcogenide sheets (or monolayers) are materials of the type MX2, with M a metal atom like molybdenum (Mo) or tin (Sn) and X a chalcogen atom like sulfur (S). The structure can be represented as a layer of X atoms on top of a layer of M atoms on top of a layer of X atoms again.
2D metal dichalcogenides belong to the so-called class of 2D materials (also including graphene), a reference to their extreme thinness. 2D materials typically have different physical properties than their bulk (3D) counterparts.
Hydrogen evolution reaction (HER), a chemical mechanism that produces hydrogen, has been explored for the electrocatalytic activity of 2D metal dichalcogenides. But now, Yasufumi Takahashi from Kanazawa University and colleagues have found that the 2D metal dichalcogenide SnS2 displays no HER catalytic activity; instead, it is a catalyst for the electrochemical reduction of carbon dioxide (CO2) to formic acid an extremely relevant property in the context of strategies for reducing the global CO2 footprint.