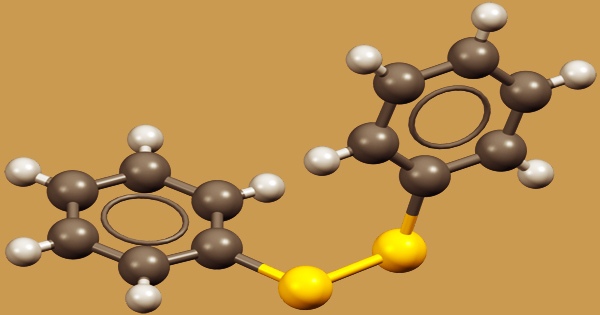
Diphenyl disulfide, often known as Ph2S2, is a white crystalline substance having the formula (C6H5S)2. It’s one among the most frequent organic disulfides used in chemical synthesis. The unpleasant odor associated with this chemical is caused by minor contamination by thiophenol. It’s most often used as a reagent for phenylthio functionality incorporation in compounds. The phenylthio group of the molecule serves a significant purpose in organic chemistry by facilitating a variety of reactions.
Under relatively moderate, normal circumstances, phenysulfides, for example, undergo oxidative elimination and produce alkenes. In radical reactions, phenyl sulfides can also be used as a source of carbon-centered radicals. The most common way to make diphenyl disulfide is to oxidize thiophenol:
2 PhSH + I2 → Ph2S2 + 2 HI
As an oxidant, hydrogen peroxide may also be employed. Because it is cheap and the precursor has an unpleasant odor, Ph2S2 is rarely produced in the laboratory. The C2S2 core of Ph2S2, like that of other organic disulfides, is non-planar, having a dihedral angle reaching 85°. Ph2S2 adds cleanly to many dienes and alkenes upon catalysis by BF3.OMe2. Ph2S2 can be used to trifluoroacetoxysulfenylate unsaturated esters, nitriles, amides, and carboxylic acids.
Ph2S2 is primarily utilized as a source of the PhS substituent in chemical synthesis. The production of PhS-substituted carbonyl compounds via the enolate is a common reaction:
RC(O)CHLiR’ + Ph2S2 → RC(O)CH(SPh)R’ + LiSPh
Thiiyl radicals can be produced from PhSSPh by thermolysis or photolysis in the presence of radical initiators. As a result, the radicals produced react reversibly with alkynes and alkenes, yielding vinyl and alkyl radicals. Ph2S2 passes through a reduction process, which is typical of disulfides:
Ph2S2 + 2 M → 2 MSPh (M = Li, Na, K)
As reductants, hydride reagents like sodium borohydride and super hydride can be employed. The strong nucleophile PhS– is found in the salts PhSM. RX (X = halide) converts most alkyl halides to thioethers with the general formula RSPh. Similarly, protonation of MSPh produces thiophenol:
PhSM + HCl → HSPh + MCl
An ammoniacal thiophenol solution is heated and a stream of air is passed over it. Dissolve the disulfide in MeOH and crystallize it. Also, repeatedly crystallize it from hot Et2O, then dry it in a vacuum at 30o over P2O5, fuse it under N2, and re-dry it; the entire operation is repeated, with a final drying under vacuum for 24 hours. Phenylsulfenyl chloride (PSCl) is formed when Ph2S2 interacts with chlorine (Zincke disulfide cleavage). Because this species is difficult to separate, it is generally produced in situ. The sulfinite ester PhS(O)OMe is formed by oxidizing Ph2S2 with lead(IV) acetate (Pb(OAc)4) in methanol.
Information Sources: