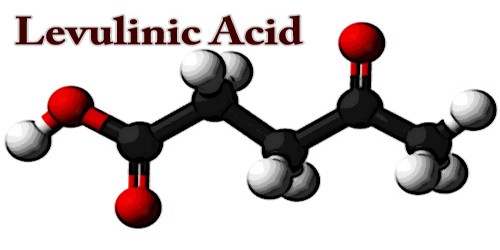
Levulinic acid (LA), or 4-oxopentanoic acid, is a crystalline keto acid prepared from levulose, inulin, starch, etc., by boiling them with dilute hydrochloric or sulfuric acids. It is an organic compound with the formula CH3C(O)CH2CH2CO2H. This white crystalline solid is soluble in water and polar organic solvents. It is derived from the degradation of cellulose and is a potential precursor to biofuels, such as ethyl levulinate.
The synthesis of LA (Levulinic acid) on the laboratory scale has been investigated extensively using homogeneous or heterogeneous catalysts. The highest reported yields of LA from monosaccharides, polysaccharides, and lignocellulosic biomass and their reaction conditions are summarized in this chapter. In addition, an overview is given on process technology studies including kinetic models and the status of large scale production of LA from biomass.
Levulinic acid is one of the key elements of the sustainable chemical industry of the future. It is used mainly in agriculture, pharmaceuticals, and cosmetics, but also to make bioplastics and biofuels.
In 1840 the Dutch professor Gerardus Johannes Mulder mentioned levulinic acid for the first time. He synthesized it by heating fructose with hydrochloric acid. The former term “levulose” for fructose gave levulinic acid its name.
Although levulinic acid has been well known since the 1870s, it has never reached a commercial use in significant volume. The first commercial production of levulinic acid began as a batch-wise process in an autoclave by A.E. Statley in the 1940s. In 1953 the US company Quaker Oats developed a continuous process for the production of levulinic acid. In 1956 it was identified as a platform chemical with high potential and in 2004 the US Department of Energy (U.S. DoE) identified levulinic acid by screening approximately 300 substances as one of the 12 potential platform chemicals in the biorefinery concept.
The acid (LA), when pure, is a white crystalline compound that has a low melting point. It is soluble in water and organic solvents like acetone and ethyl acetate. Each molecule has two functional groups an acid carboxyl and keto carbonyl. These groups make the compound valuable as a precursor for conversion to a variety of commercially attractive derivatives. A notable example is methyltetrahydrofuran which is used as a fuel additive and a solvent.
Levulinic acid derivatives and their application will be presented along with future prospects of LA synthesis in biorefineries. Many concepts for the commercial production of levulinic acid are based on a strong acid technology. Pure levulinic acid is isolated by evaporation of the extraction solvent and distillation of the levulinic acid. Companies who developed technology based on this concept include Biofine, DSM, Segetis, and GFBiochemicals. GFBiochemicals started the commercial production of levulinic acid in 2015 at a production scale of 2,000 MT/a in Caserta, Italy. 2Caserta is the world’s largest operational production plant for levulinic acid.
Commercial production of levulinic acid (LA) from lignocellulose involves shredding the source material so that it can readily be treated to produce monosaccharide sugars like glucose and fructose. The process requires the use of a dilute mineral acid such as hydrochloric acid or sulfuric acid. The acid acts as a catalyst and is recycled. The sugars are then converted in a two-step process to levulinic acid and formic acid. Fermentation processes are also being developed to produce levulinic acid and its derivatives from biomass.
Levulinic acid (LA) is relatively nontoxic, with an LD50 of 1850 mg/kg. It is used as a precursor for pharmaceuticals, plasticizers, and various other additives. The largest application of levulinic acid is its use in the production of aminolevulinic acid, a biodegradable herbicide used in South Asia.
Levulinic acid is a platform chemical with significant potential to replace petroleum-based products in the chemical and biofuel sectors. Another key application is the use of levulinic acid in cosmetics. LA is also used in cigarettes to increase nicotine delivery in the smoke and the binding of nicotine to neural receptors.
Information Sources: