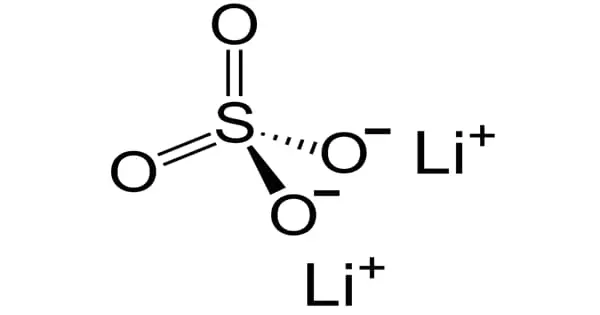
Lithium sulfate, also known as Li2SO4, is a white inorganic salt with the formula Li2SO4. It is a colorless crystalline solid that can be monoclinic or hexagonal. It is the sulfuric acid lithium salt. It is a metal sulfate with lithium as the counterion and a lithium-to-sulfate ratio of 2:1. It functions as an antidepressant. It has a lithium (1+) content.
Despite the fact that lithium sulfate solution is produced in large quantities as an intermediate in the spodumene-sulfuric acid process, the pure compound has limited industrial applications. It is used in small amounts in molten salt baths to chemically strengthen the glass. It is also used to treat manic depression, among other things.
Physical properties
Lithium sulfate is soluble in water, but it does not follow the usual trend of increasing solubility with the temperature that most salts do. On the contrary, as its dissolution is an exothermic process, its solubility in water decreases with increasing temperature. This unusual property, also known as retrograde solubility, is shared by a few inorganic compounds, including calcium hydroxide, calcium sulfates, and lanthanoid sulfates, all of which have exothermic dissolution reactions.
- Molecular Weight: 109.945
- Appearance: White
- Melting Point: 859° C (1,578° F)
- Boiling Point: 1,377° C (2,511° F)
- Density: 2-C.22 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 109.984
Because they are piezoelectric, lithium sulfate crystals are also used in ultrasound-type non-destructive testing because they are very efficient sound receivers. They do, however, suffer in this application due to their water solubility.

The most common form of lithium sulfate is lithium sulfate monohydrate, which has hygroscopic properties. Anhydrous lithium sulfate has a density of 2.22 g/cm3, but weighing lithium sulfate anhydrous can be difficult because it must be done in a water-free environment.
Pyroelectric properties are possessed by lithium sulfate. The electrical conductivity of aqueous lithium sulfate increases when it is heated. The electrical conductivity is also affected by the molarity of lithium sulfate; optimal conductivity is achieved at 2 M and then decreases.
Crystal properties
Lithium sulfate has two distinct crystal phases. In its common phase II form, lithium sulfate has a sphenoidal monoclinic crystal system with edge lengths of a = 8.23Å b = 4.95Å c = 8.47Å β = 107.98°. When lithium sulfate is heated above 130°C, it changes to a water-free state while retaining its crystal structure. It is not until 575°C that the transition from phase II to phase I occurs. The crystal structure shifts to a face-centered cubic crystal system with an edge length of 7.07Å. During this phase transition, the density of lithium sulfate decreases from 2.22 to 2.07 g/cm3.
Preparation
Lithium sulfate is prepared by neutralization of lithium hydroxide or lithium carbonate with sulfuric acid followed by crystallization:
2LiOH + H2SO4 → Li2SO4 + H2O
Li2CO3 + H2SO4 → Li2SO4 + CO2 + H2O
The product obtained from crystallization in a concentrated solution is the monohydrate, Li2SO4•H2O. The anhydrous salt is obtained by heating the monohydrate in a vacuum.
Reactions
Endothermic disassociation occurs when solid lithium sulfate is dissolved in water. Sodium sulfate, on the other hand, has an exothermic disassociation. However, the exact energy of disassociation is difficult to quantify because it appears to be affected by the amount (number of mols) of salt added to water. Small amounts of dissolved lithium sulfate cause far more temperature change per mol than large amounts.
Uses
Lithium sulfate is being investigated as a possible component of ion-conducting glasses. The transparent conducting film is a hotly debated topic because it is used in applications such as solar panels and has the potential to create a new class of batteries. It is critical to have a high lithium content in these applications; the more commonly known binary lithium borate (Li2O• B2O3) is difficult to obtain with high lithium concentrations and difficult to keep because it is hygroscopic.
The addition of lithium sulfate to the system allows for the formation of an easily produced, stable, high lithium concentration glass. The majority of today’s transparent ionic conducting films are made of organic plastics, and it would be ideal if a low-cost stable inorganic glass could be developed.
Lithium sulfate has been successfully tested as an additive for Portland cement to accelerate curing. Lithium sulfate accelerates the hydration reaction, reducing curing time. The strength of the final product is a concern with reduced curing time, but when tested, lithium sulfate doped Portland cement showed no observable decrease in strength.