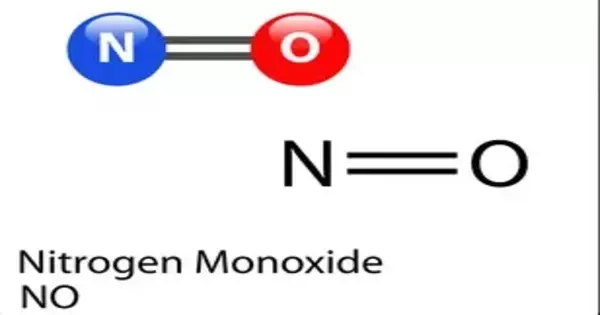
Nitric oxide (NO), also known as nitrogen monoxide, is a colorless gas with the chemical formula NO. It is a diatomic molecule with one nitrogen and one oxygen atom. Nitric oxide is an important signaling molecule in the human body that participates in a variety of physiological processes.
It functions as a signaling molecule in the human body and is important in a variety of physiological processes such as vasodilation (blood vessel widening), neurotransmission, and immune response regulation.
Properties
- Molecular weight: 30.01 g/mol
- Appearance: It is a colorless gas at room temperature and pressure.
- Odor: It has a sharp, sweetish odor.
- Melting point: It does not have a solid state at atmospheric pressure. It condenses to a liquid at temperatures below -152 degrees Celsius (-242 degrees Fahrenheit).
- Boiling point: It is a gas at room temperature, and it boils at -151.7 degrees Celsius (-241.1 degrees Fahrenheit).
- Density: The density of nitrogen monoxide gas is about 1.34 g/L.
- Solubility: It is sparingly soluble in water, and its solubility increases with lower temperatures.
Chemical properties
Nitric oxide is a free radical gas with a brief half-life. It is extremely reactive and easily interacts with other molecules in biological systems. It is produced enzymatically in the human body by a group of enzymes known as nitric oxide synthases (NOS). It is created by combining the amino acid L-arginine and molecular oxygen.
Nitric oxide is a highly reactive gas that, when combined with oxygen, produces nitrogen dioxide (NO2). It also combines with other molecules and radicals in the atmosphere, contributing to atmospheric chemistry.
Biological functions
Nitric oxide functions as a signaling molecule in a variety of physiological processes, including vasodilation (blood vessel widening), blood pressure regulation, neurotransmission, immune response, and cell signaling.
It controls blood vessel tone and dilation, increasing blood flow and lowering the risk of cardiovascular disease. It prevents blood clots and plaque buildup by inhibiting platelet aggregation and smooth muscle cell proliferation. It functions in the central nervous system as a neurotransmitter. It contributes to neuronal communication, synaptic plasticity, and memory formation.
Industrial applications
Various industrial processes make use of nitric oxide. It is used to make nitric acid, which is used to make fertilizers, explosives, and other chemicals. Nitric oxide is also used to reduce emissions in combustion processes, such as car engines.
In high concentrations, nitric oxide is toxic. It can combine with hemoglobin in the blood to form methemoglobin, which reduces the ability of the blood to transport oxygen. Prolonged exposure to high NO levels can cause health problems.