Chemical bonds are what join atoms together. They are the connections between atoms in a molecule. They are forces that hold atoms together to make compounds or molecules. Atoms bonded stay together unless the needed amount of energy is transferred to the bond. It describes a variety of interactions that hold atoms together in chemical compounds.
In general, strong chemical bonding comes with the sharing or transfer of electrons between the participating atoms. The atoms in molecules, crystals, metals and diatomic gases are held together by chemical bonds.
There are two types of bonds; covalent and ionic. Covalent bonds form when atoms share electrons. Ionic bonding is the attraction between oppositely charged ions. Atoms with large differences in electronegativity transfer electrons to form ions. The ions then are attracted to each other. This attraction is known as an ionic bond. A covalent bond is an interaction between two atoms, which involves the sharing of one or more electrons to help each atom satisfy the octet rule. This interaction typically forms between two non-metals. Metallic bonds are formed between valence electrons and the metal atoms. Other types of bonds include metallic bonds and hydrogen bonding. Chemical bonds are negatively charged electrons that are pulling protons into each other.
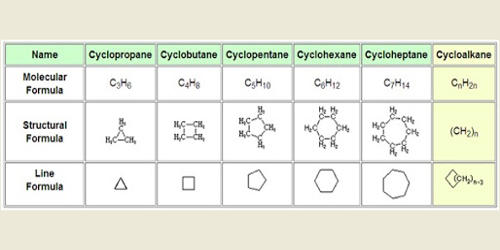
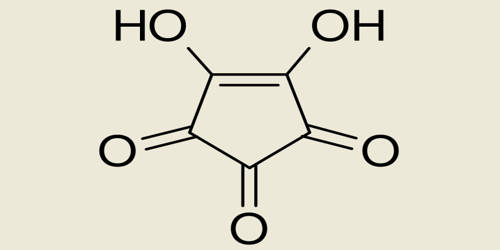
Because atoms and molecules are three-dimensional, it is difficult to use a single method to indicate orbitals and bonds. In molecular formulas, the chemical bonds between atoms are indicated in different ways depending on the type of discussion.
A common way chemists describe chemical bonds is through the number of electrons each atom has on itself. Each atom is drawn with the number of electrons as dots or lines to form a maximum of eight. If the electrons form a chemical bond then a line is drawn between the two electrons. The number of bonds developed increases the number of lines.