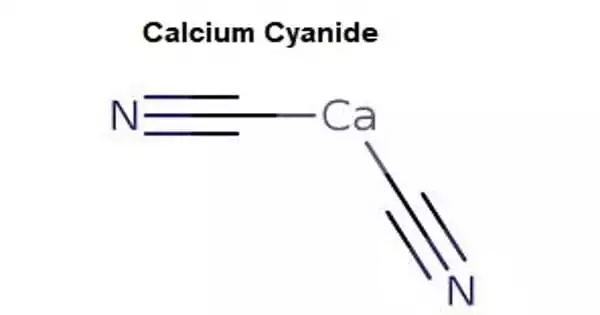
Amygdalin (from Ancient Greek: ἀμυγδαλή amygdálē “almond”) is a cyanogenic glucoside found in almonds and the seeds of other Rosaceae plants. It’s a naturally occurring chemical molecule that’s best known for being advertised as a cancer cure when it wasn’t. It can be found in the seeds (kernels) of apricots, bitter almonds, apples, peaches, and plums, among other plants. Amygdalin is metabolized to benzaldehyde, D-glucose, and hydrocyanic acid by plant emulsin (a glucosidase/nitrilase complex) or hydrochloric acid.
After eating the bitter almond, Amygdalin releases free cyano groups, poisoning the food. It’s a byproduct of the bitter almond’s phenylalanine metabolism. Because each amygdalin molecule contains a nitrile group, which can be released as the poisonous cyanide anion by the action of a beta-glucosidase, it is categorized as a cyanogenic glycoside. Consumption of amygdalin causes the release of cyanide in the human body, which can result in cyanide poisoning.
Amygdalin is an amygdalin with an R-configuration stereocentre on the cyanohydrin function. Both amygdalin and a chemical derivative known as laetrile have been pushed as alternative cancer remedies since the early 1950s, frequently under the misnomer vitamin B17 (neither amygdalin nor laetrile is a vitamin). Amygdalin contains the enzymes -glucosidase and amygdalinase (oxynitrilase), which hydrolyze amygdalin to produce two molecules of glucose and one molecule of amygdalenone; the latter hydrolyzes mandelonitrile to produce almond nitrile cyanide (HCN) and benzaldehyde.
Amygdalin is found in seeds like almonds. It functions as a plant metabolite, an inducer of apoptosis, and an anticancer agent. Agents derived from higher plants that have been shown to have cytostatic or anti-cancer properties. It is made up of (R)-mandelonitrile. They were proven to be clinically unsuccessful in the treatment of cancer in a scientific investigation, as well as possibly dangerous or deadly when taken by mouth due to cyanide poisoning.
Stone fruit kernels, such as almonds, apricots (14 g/kg), peaches (6.8 g/kg), and plums (4–17.5 g/kg depending on type), as well as apple seeds (3 g/kg), contain amygdalin. Glycosides are found in many plant root cells, and when they are in the sugar type, they have no harmful effects. Hydrolysis of glycosides produces poisonous chemicals that cause cell death. Mandelic glycosides are a type of glycoside found in peach root. Bitter almond amygdalin concentrations ranged from 33 to 54 g/kg depending on variety; semibitter kinds averaged 1 g/kg, while sweet varieties averaged 0.063 g/kg, with significant variation depending on variety and growing region, according to one study.
Hydrogen cyanide and benzaldehyde are produced when amylose is hydrolyzed. The chiral phenyl center of natural amygdalin has the (R) configuration. This stereogenic center isomerizes at mild basic circumstances, and the (S)-epimer is known as neoamygdalin. Peeling root lesion development and necrosis occurred near the stabbing nematode but not in the contact zone, and in vitro experiments revealed that the pratylenchus penetrans can hydrolyze amygdalin.
As a cyanide-containing glucoside, amygdalin can be utilized to identify, differentiate, and characterize enzymes such maltase, almond casein, and -glucosidase. Laetrile, which is mostly made up of amydgalin, a glycoside derived from the kernels of apricots, peaches, and other fruits, has been available in cancer-treatment formulations for more than 30 years. Despite the lack of proof that these are effective, preparations were frequently used and, until the late 1970s, were thought to be innocuous.
Information Sources: