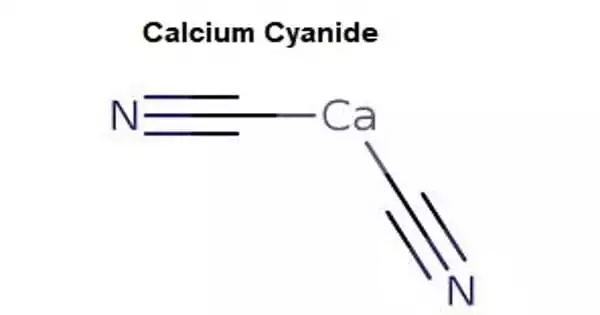
Calcium cyanide, also known as black cyanide, is an inorganic compound with the formula Ca(CN)2 that is the calcium salt of hydrocyanic acid. It appears in the form of white crystals, powder, or gray-black powder. Although rare, the pure form is a white solid; commercial samples can be black-gray. It easily hydrolyzes (even in moist air) to release hydrogen cyanide. It is extremely toxic, as are other cyanides of a similar nature. It is toxic when absorbed through the skin through open wounds, ingested, or inhaled.
Cyanides are primarily used in the steel, electroplating, mining, and chemical industries. The most common cyanide compounds used in industrial processes are potassium and sodium cyanide, as well as calcium cyanide, especially in metal leaching operations.
Properties
- Melting point: 640 estimated
- Density: 1.8 g/cm3
- Form: white rhombohedral crystals
- Water Solubility: soluble H2O, gradually releasing HCN
Preparation
Calcium cyanide can be made by treating powdered calcium oxide with boiling anhydrous hydrocyanic acid in the presence of an accelerator such as ammonia or water to reduce hydrocyanic acid loss during polymerization.
It can also be made by combining liquid hydrocyanic acid and calcium carbide. Calcium cyanide can also be made by reacting hydrocyanic acid gas with quicklime (CaO) at high temperatures near 400 °C. Calcium cyanimide is formed instead at higher temperatures, around 600 °C. The material is frequently contaminated with polymeric derivatives of hydrogen cyanide, which results in black color.

Production Methods
Commercially, calcium cyanide is produced from lime, calcium oxide, coke, and nitrogen. The reactions take place in an electric furnace. To prevent reversion to calcium cyanamide, the resulting melt is rapidly cooled. Because of the presence of carbon, the product is marketed in the form of flakes that are dark gray. The extraction or cyanidation of precious metal ores was the first and continues to be the most important application for calcium cyanide.
Reactivity
Calcium cyanide easily hydrolyzes to form hydrogen cyanide gas. Because of the presence of an acid, the evolution of hydrogen cyanide gas was accelerated. It is reactive toward oxidizing agents. By reacting calcium cyanide with ammonium carbonate, ammonium cyanide is sometimes produced.
Ca(CN)2 + (NH4)2CO3 → 2 NH4CN + CaCO3
Uses
Calcium cyanide is primarily used to extract or cyanidate gold and silver ores. It is also used to make prussiates or ferrocyanides, in mineral froth flotation, in processes where gold complexes are adsorbed on carbon, in the production of stainless steel, as a fumigant and rodenticide, and as a cement stabilizer.
Calcium cyanide is almost entirely used in the mining industry. It is used as a low-cost cyanide source in many leaching or vat operations to extract precious metals such as gold and silver from their ores. It accomplishes this by forming coordination complexes with the metals, allowing them to be separated from the ores. It is distributed in either solid flake form or in liquid form.
Toxicity
The high toxicity of calcium cyanide to touch, inhale, or ingest makes it useful as a rodenticide. It has, for example, been used to manage the population of Indian crested porcupines (Hystrix indica). Its toxicity has been used as an insecticide in the same way. However, because of its high toxicity, it is unfavorable in many cases, and other less harmful chemicals are frequently used instead. It is also used in the production of hydrogen cyanide, ammonium cyanide, and ferrocyanides.