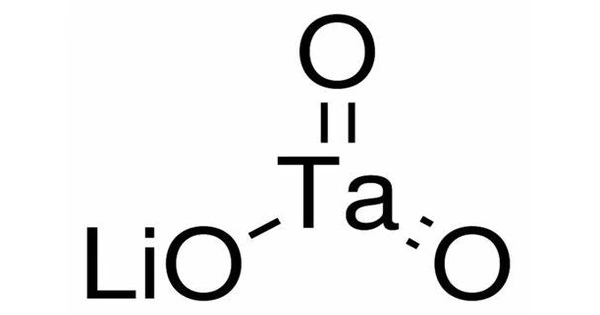
Lutetium tantalate, also known as LuTaO4, is a chemical compound composed of lutetium, tantalum, and oxygen. It is made up of lutetium (Lu), tantalum (Ta), and oxygen (O). This salt is the densest known white stable material, with a density of 9.81 g/cm3. It is a tantalate, which are compound that contains tantalum in combination with oxygen and other elements.
Tantalum is a transition metal and lutetium is a rare earth element. Tantalates, including lutetium tantalate, can have interesting properties and are frequently researched for potential applications in fields such as materials science and electronics. (Although thorium dioxide ThO2 is also white and has a higher density of 10 g/cm3, it is radioactively unstable; while not radioactive enough to make it unstable as a material, even its low rate of decay is still too much for certain uses such as phosphors for detecting ionizing radiation.)
Properties
LuTaO4 has a monoclinic (labeled as M’; Pearson symbol mP12, Space group = P2/a, No 13) fergusonite-type crystal structure under standard conditions. By annealing at 1,600 °C, this can be converted to an I2/a (M) structure. Under normal conditions, both structures are stable. The lutetium atom is 8-fold coordinated with oxygen in the M’ structure, forming a distorted antiprism with C2 site symmetry. Lutetium tantalate has the same structure as yttrium tantalate (YTaO4) and gadolinium tantalate (GdTaO4).
LuTaO4’s white color and high density make it ideal for phosphor applications, though the high cost of lutetium is an impediment. The properties and applications of lutetium tantalate can be affected by its crystal structure and composition.
- Optical Properties: Because of its favorable optical properties, it is frequently used in optical applications. It has a broad bandgap, which allows it to be transparent in the visible and near-infrared regions of the electromagnetic spectrum.
- Dielectric Properties: It has a high dielectric constant, making it useful in applications like capacitors. The dielectric constant expresses a material’s ability to store electrical energy in the presence of an electric field.
Preparation
Powders of lutetium and tantalum oxides (Lu2O3 and Ta2O5) are mixed and annealed at temperatures above 1,200 °C for several hours to prepare a sample of lutetium tantalate. Before annealing, a small fraction of an appropriate material, such as an oxide of another rare-earth metal, is added to the mixture to make a phosphor. After cooling, the product is leached with water, washed, filtered, and dried, yielding a white powder containing micrometer-sized LuTaO4 particles.