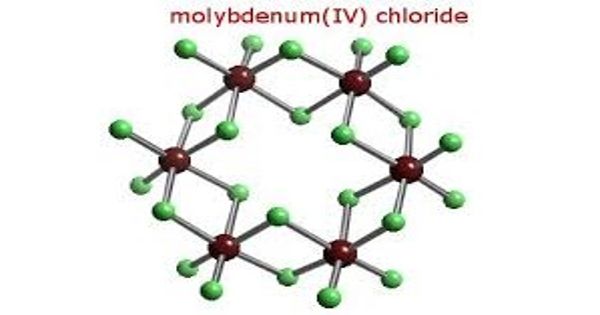
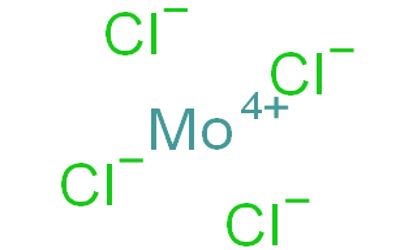
Molybdenum tetrachloride is the inorganic compound with the empirical formula MoCl4. This thermally unstable, dark green solid is used to prepare other complexes of molybdenum. The material exists as two polymorphs, a polymeric (“α”) and a hexameric (“β”) structure, although neither form is soluble in any solvent without degradation. In each polymorph, the Mo center is octahedral with two terminal chloride ligands and four doubly bridging ligands. In a hydrogen atmosphere at 230–300°C, gaseous MoCl4 does not react with the hydrogen but disproportionates. Above 400°C, gaseous MoCl4 reacts with hydrogen before undergoing disproportionation.
Molybdenum trichloride is successfully prepared in quantity by the reduction of molybdenum pentachloride with hydrogen. This thermally unstable, dark green solid is used to prepare other complexes of molybdenum.
Molybdenum tetrachloride is prepared by direct reaction of molybdenum trichloride with molybdenum pentachloride in a sealed tube or steel bomb maintained at 250°C. X-ray patterns of the various chlorides were obtained.
Properties
Gaseous MoCl4 is stable above 600°C. It disproportionates below 500°C, giving MoCl2.9 at 500°C and MoCl3.0 at 400-230°C. The percentage of gaseous MoCl4 which disproportionates increases with decreasing temperature.
- Compound Formula: MoCl4
- Molecular Weight: 253.75 g/mol
- Appearance: Green Hygroscopic Powder
- Melting Point: 101°C
- Boiling Point: N/A
- Density: N/A
- Average mass: 237.752 Da
- Monoisotopic mass: 237.780823 Da
- Major Category: Metals
Preparation
Molybdenum trichloride is successfully prepared in quantity by the reduction of molybdenum pentachloride with hydrogen. The most satisfactory yields were obtained with a 4 to 5 mole excess of hydrogen at pressures of 100 psi or higher and at a temperature of 125°C.
It can be prepared from by dechlorination of molybdenum pentachloride using tetrachloroethene:
2 MoCl2 + C2Cl4 → 2 MoCl4 + C2Cl6
The acetonitrile complex adduct, which is a versatile intermediate, can be prepared directly from the pentachloride:
2 MoCl5 + 5 CH3CN → 2 MoCl4(CH3CN)2 + ClCH2CN + HCl
The MeCN ligands can be exchanged with other ligands:
MoCl4(CH3CN)2 + 2 THF → MoCl4(THF) + 2 CH3CN
Information Source: