The anomeric effect is a concept in organic chemistry that addresses the stereoelectronic effects influencing the conformation and reactivity of functional groups at the anomeric carbon atom of saccharides (sugar molecules), notably in cyclic forms such as pyranoses and furanoses. When a sugar molecule undergoes intramolecular cyclization to produce a ring structure, such as glucose or fructose rings, the anomeric carbon atom becomes a component of a new sigma bond.
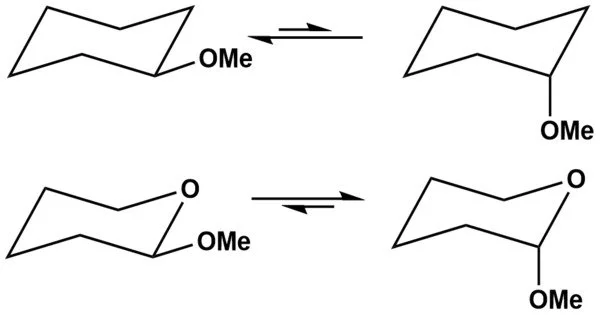
The anomeric effect, also known as the Edward-Lemieux effect, is a stereoelectronic effect in organic chemistry that describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the axial orientation over the less hindered equatorial orientation expected from steric considerations. J. T. Edward discovered this phenomenon in pyranose rings while investigating carbohydrate chemistry in 1955.
At the anomeric carbon, there are two potential configurations: alpha and beta anomers. These are distinguished by the spatial arrangement of substituents at the anomeric carbon atom and are commonly symbolized by the Greek letters α and β. The anomeric effect explains why one conformation is preferred over another and why these anomers are stable.
The term “anomeric effect” was coined in 1958. The phrase “anomeric carbon” refers to the lowest-numbered ring carbon of a pyranose. Anomers are isomers that differ only in their conformation at the anomeric carbon. D-glucopyranose anomers are diastereomers, with the beta anomer having an OH group pointing up equatorially and the alpha anomer having that OH group pointing down axially.
Key points about the anomeric effect include:
- Alpha and Beta Anomers: When a sugar molecule forms a cyclic ring structure, the position of the hydroxyl group (-OH) attached to the anomeric carbon can either be in the axial (α) or equatorial (β) position. This distinction arises from the interaction of lone pairs of electrons on the oxygen atom of the hydroxyl group with the sigma bond formed upon ring closure.
- Axial Preference: The anomeric effect predicts that the axial position is less crowded and, therefore, more stable. This results in the α-anomer being less stable than the β-anomer. As a consequence, the β-anomer is often the more prevalent and thermodynamically favored form.
- Influence on Reactivity: The anomeric impact influences the reactivity of the sugar molecule as well. Because of its higher energy, the axial orientation is often more reactive, which can be essential in glycosidic bond formation and other chemical processes involving sugars.
In conclusion, the anomeric effect is critical in influencing the conformation, stability, and reactivity of sugar molecules in their cyclic forms. It is a key topic in carbohydrate chemistry and has ramifications in a variety of biological activities, including protein and enzyme recognition and binding of sugars.