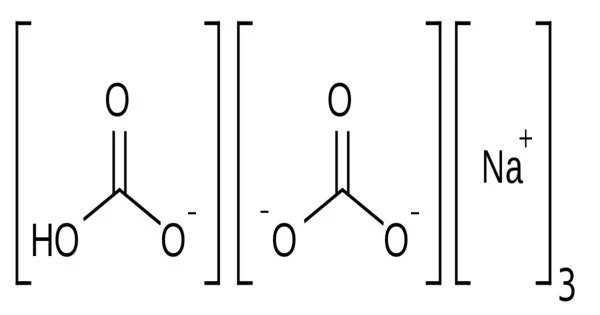
Sodium Sesquicarbonate is a chemical compound with the chemical formula Na2CO3·1.5H2O. It is also known as sodium sesquicarbonate monohydrate and is a white crystalline powder that is commonly used as a cleaning and water-softening agent.
In cleaning applications, sodium sesquicarbonate can be used as a component in household and industrial cleaning products, as well as in laundry detergents. In water softening applications, sodium sesquicarbonate can be used to remove hard water ions, such as calcium and magnesium, from water to improve its quality.
Properties
It has a needle-like crystal structure and is a double salt of sodium bicarbonate and sodium carbonate (NaHCO3 • Na2CO3). However, the term is also applied to a powdered equimolar mixture of those two salts with whatever water of hydration the sodium carbonate contains.
The evaporite mineral trona contains the dihydrate, Na3H(CO3)2 • 2H2O. Because of concerns about the toxicity of borax, which was withdrawn as a cleaning and laundry product, sodium sesquicarbonate is marketed as a “Borax substitute” in the European Union (EU). It is also referred to as one of the E500 food additives.
- Chemical formula: Na3H(CO3)2·2H2O
- Appearance: white, needle-like
- Density: 2.112 g/cm3 (dihydrate)
- Solubility in water: dehydrate 13 g/100 mL (0 °C); 42 g/100 mL (100 °C)
- Crystal structure: monoclinic (dihydrate)

Uses
Sodium sesquicarbonate is used in bath salts, and swimming pools, as an alkalinity source in water treatment, and as a phosphate-free product that replaces trisodium phosphate in heavy-duty cleaning. It is used to preserve copper and copper alloy artifacts that corrode when exposed to salt (called “bronze disease” due to its effect on bronze). Copper(I) chloride is formed when salt chloride reacts with it.
It is also used as a precipitating water softener, combining with hard water minerals (calcium and magnesium-based minerals) to form an insoluble precipitate, thereby removing these hardness minerals from the water. The precipitate is formed by the carbonate moiety, with bicarbonate added to moderate the material’s alkalinity.
It is important to note that while sodium sesquicarbonate is generally considered to be a safe and effective cleaning and water-softening agent, it is always important to follow the manufacturer’s instructions and to use it in accordance with applicable safety regulations.