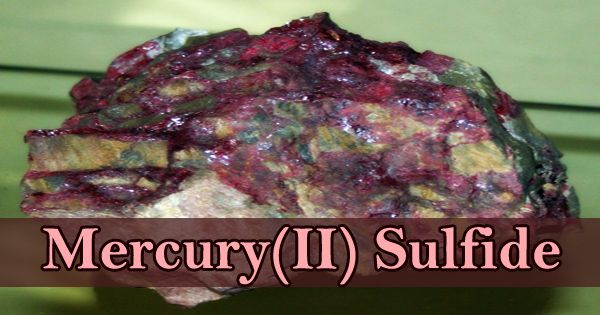
The odorless red or black solid appears to be mercury sulfide, mercuric sulfide, mercury sulphide, or mercury(II) sulfide; sinks in water, and insoluble in water. It is a chemical compound consisting of mercury and sulfur, the chemical elements. It is represented by the chemical formula HgS. Mercury(II) sulfide has several modifications; the red hexagonal form is known as cinnabar (alpha form) and the black cubic modification (beta form) are the only two stable allotropic forms. Mercury sulfide (HgS) is a fine, very brilliant scarlet powder which if ingested, is deadly. Often known as the cinnabar and metacinnabar of mercury ore, it is used as a pigment in paint manufacturing.
The type in which mercury is most commonly found in nature is red cinnabar (α-HgS, trigonal, hP6, P3221). It is a red crystalline or powdery substance; hexagonal crystal system; refractive index 2.854; density 8.10 g/cm3; sublime at 583.5°C; color changes to brown at 250oC and transforms to black sulphide at 386°C; returns to cooling red color; insoluble in water, alcohol and nitric acid; soluble in aqua regia and alkali metal sulphide solutions; decomposed by hot condensed sulphuric acid.

(Example of Mercury(II) Sulfide)
Black metacinnabar (β-HgS), is less natural in nature and adopts the crystal structure of zinc blende (T2d-F43m). It is a black amorphous powder or crystalline material (beta form); cubic structure; metastable at normal temperatures; converted to red sulphide at normal pressure by sublimation; 7.73 g/cm3 density; melted at 583.5°C; insoluble in water, alcohol, and nitric acid; soluble in aqua regia, alkali and alkali metal sulphide solutions.
When H2S is bubbled by solutions of Hg(II) salts, Beta (β)-HgS is precipitated as a black powder. Of all but concentrated acids, β-HgS is unreactive. Red sulfide occurs naturally and is derived from cinnabar minerals. It can also be prepared by heating mercury with a potassium pentasulfide solution, forming scarlet cinnabar:
Hg + K2S5 → HgS + K2S4
Red sulfide can also be made from black sulfide by heating the alkaline polysulfide in a concentrated solution. The pigment shade varies with the black sulfide temperature, reaction time, and concentration. Mercury metal is produced by roasting in the air and condensing the vapor from the cinnabar ore. The sulfide of black mercury(II) is normally prepared with hydrogen sulfide by precipitation from an aqueous solution of mercury(II) salt. Thus a pale yellow precipitate of the HgCl2•2HgS composition forms when H2S is passed into a solution of HgCl2.
Other processes, such as adding sodium thiosulfate, Na2S2O3 in excess of a dilute solution of sodium mercuric chloride, Na2HgCl4, and treating mercury with molten or powdered sulfur, can also be used to create black sulfide. It is known as vermilion when α-HgS is used as a red pigment. Conversion from red α-HgS to black β-HgS has been correlated with the propensity of vermilion to darken. The metallic mercury from these setups is processed into mercury sulfide for underground storage as the mercury cell as used in the Chlor-alkali industry (Castner-Kellner process) is being phased out over concerns about mercury pollution.
Inhaling dust levels of 1.2-8.5 mg/m 3 in the air may result in acute poisoning; symptoms include chest pain and tightness, coughing, and trouble breathing. If ingested, toxicity depends on the release of the Hg ++ ion; kidney, mental, and nervous disorders can be caused by chronic mercury poisoning. Dust irritates the eyes and induces allergic dermatitis frequently; systemic poisoning can be triggered by skin absorption. Unique Risks of Combustion Products: Toxic mercury vapor and irritating sulfur dioxide gas are found in fire smoke. Fire behavior: Changes color when heavy. At burning temperature, it decomposes. The black type may soften, and it may flow out and burn molten sulfur.
Information Sources: