Hafnium carbide (HfC) is a chemical compound of hafnium and carbon that has a melting temperature of roughly 3900oC, making it one of the most refractory binary compounds known to man. It is a hafnium-carbon chemical compound that is one of the most refractory binary compounds known. The reduction of hafnium oxide with carbon at 1800 to 2000°C produces hafnium carbide powder, which requires a considerable processing time to remove all oxygen.
It does, however, have a limited resistance to oxidation, with oxidation beginning at temperatures as low as 430°C. This chemical may be used as a heat shield on future spacecraft. It has the greatest melting point of any binary alloy known, allowing it to be used in a wide range of high-temperature applications. It’s being studied for use in high-temperature applications including rocket nozzles and scramjet components.
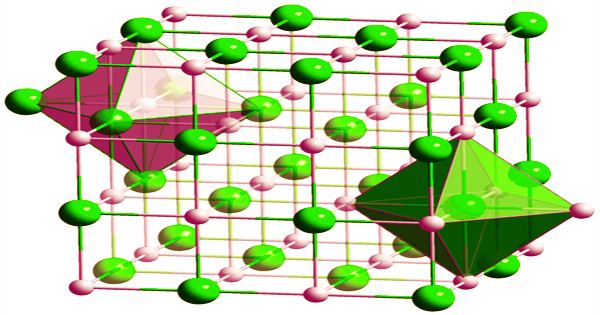
A portion formed of Hafnium carbide fiber matrix composite may theoretically withstand temperatures that would soften or melt even refractory metal-metal alloys like rhenium-tungsten blends. Because HfC is often carbon-deficient, its composition is frequently written as HfCx (x = 0.5 to 1.0). At any value of x, it possesses a cubic (rock-salt) crystal structure. Because it has a density of around 60% that of metal alloys, essential rocket, missile, and aerospace components manufactured with HfC composites would be significantly lighter.
Hafnium carbide is also utilized in hard coatings, which are commonly deposited using plasma spraying. HfC structural foams can also be utilized as a thermal insulation material or built into high-temperature components. It’s also used in the ceramics business. The reduction of hafnium(IV) oxide with carbon at 1800 to 2000 °C yields HfC powder. To eliminate all oxygen, a significant processing period is required.
The fabrication process of HfC ceramic fibers is relatively complex: It is based on melting a preceramic polymer containing hafnium, extruding that polymer through an orifice to form fiber, cross-linking that fiber, and heating the cross-linked fiber at a temperature greater than 600°C under controlled atmospheric conditions to obtain a hafnium carbide containing ceramic fibre. Chemical vapor deposition using a gas mixture of methane, hydrogen, and vaporized hafnium(IV) chloride can also be used to make high-purity HfC coatings.
Hafnium carbide is a brittle, dark gray solid. It is made by heating a mixture of components or by reacting hafnium tetrachloride with methane at 2100oC. During the large-scale manufacturing of pure zirconium for nuclear reactors, sufficient quantities of hafnium oxide or hafnium metal sponge are obtained. Despite its advantageous features such as high hardness (>9 Mohs) and melting point, HfC has a relatively restricted use due to its technical complexity and high cost of manufacture.
HfC composites could be an intriguing choice for increasing combustion chamber working temperatures. HfC composite liners may be useful in the construction of reusable rocket engines for both launcher and spacecraft applications due to their zero-ablation features. HfCx‘s magnetic characteristics vary from paramagnetic to diamagnetic as x increases. Despite having the same crystal structure as HfCx, TaCx exhibits an inverted characteristic (dia-paramagnetic transition with increasing x).
On a large scale, hafnium carbide can be made by carburizing in vacuo in the presence of hydrogen from hydrided hafnium sponge at 1500–1700 °C or from hafnium oxide at 2000–2200 °C. Bonding, structural failure under mechanical pressure, vibration, acoustic noise, and thermal expansion/cycling conditions are some of the constraints and important technical difficulties connected with HfC ceramic fibre matrix material in general and specifically as a rocket chamber/nozzle liner. The resulting hafnium carbide is more like a carbon solution at specified interstitial sites of a face-centered cubic hafnium lattice than a true stoichiometric compound.
At room temperature, hafnium carbide is inert to most reagents, but hydrofluoric acid solutions dissolve it. At 250–500 °C, hafnium carbide interacts exothermally with halogens to generate hafnium tetrahalide, and at temperatures over 500 °C, it interacts exothermally with oxygen to generate hafnium oxide. At higher temperatures, hafnium carbide slowly loses some of its carbon in the presence of hydrogen. Assessment of the feasibility, benefits, and downsides of employing HfC composites in other spaceship components with extremely high thermal loads.
Information Sources: