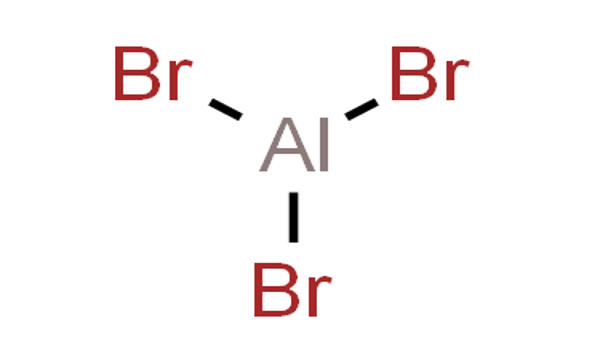
Aluminium bromide is any chemical compound with the empirical formula AlBrx. It appears as a light-yellow-colored liquid. Aluminium tribromide is the most common form of aluminium bromide. In its solid-state, it is colorless crystals that quickly absorb water causing old samples to be highly hydrated. It is a colorless, sublimable hygroscopic solid; hence old samples tend to be hydrated, mostly as aluminium tribromide hexahydrate (AlBr3·6H2O).
Properties
- Molecular Weight: 266.69
- Appearance: White/pale pink solid
- Melting Point: 97.8 °C
- Boiling Point: 265 °C
- Density: 3.205 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 265.734503
- Monoisotopic Mass: 263.73655
Structure
Aluminum bromide is a product of the reaction of aluminum and bromine combined in a 1 to 3 ratio to produce a neutral compound. The dimeric form of aluminum tribromide (Al2Br6) predominates in the solid-state, in solutions in noncoordinating solvents (e.g. CS2), in the melt, and in the gas phase. Only at high temperatures do these dimers break up into monomers:
Al2Br6 → 2 AlBr3 ΔH°diss = 59 kJ/mol
The species aluminium monobromide forms from the reaction of HBr with Al metal at high temperature. It disproportionates near room temperature:
6/n “[AlBr]n” → Al2Br6 + 4 Al
This reaction is reversed at temperatures higher than 1000 °C. Aluminium monobromide has been crystallographically characterized in the form the tetrameric adduct Al4Br4(Net3)4 (Et = C2H5). This species is electronically related to cyclobutane. The mixing of aluminum bromide with chlorine produces aluminum chloride and bromine.
Al2Br6 consists of two AlBr4 tetrahedra that share a common edge. The molecular symmetry is D2h.
The monomer AlBr3, observed only in the vapor, can be described as trigonal planar, D3h point group. The atomic hybridization of aluminium is often described as sp2. The Br-Al-Br bond angles are 120°.
Synthesis
By far the most common form of aluminum bromide is Al2Br6. This species exists as a hygroscopic colorless solid at standard conditions. It appears as a white to yellowish-red, lumpy solid with a pungent odor. Typical impure samples are yellowish or even red-brown due to the presence of iron-containing impurities. The main characteristic of aluminum bromide is its ability to absorb water, alcohol, carbon sulfide, and acetone. It is prepared by the reaction of HBr with Al:
2 Al + 6 HBr → Al2Br6 + 3 H2
Alternatively, the direct bromination occurs also:
2 Al + 3 Br2 → Al2Br6
Uses
Aluminum bromide has very little commercial use but does electroplate aluminum to steel producing a smooth, thick, adherent, and shiny finish. Using aluminum bromide to do electroplating has been found to be better than using aluminum chloride because it forms a stronger bond with the steel.
Precaution
It is very corrosive to skin, eyes, and mucous membranes. As a liquid, it is a very corrosive yellow that is damaging to the skin, eyes, and mouth. It is used to make other chemical substances.
Informtion Source: