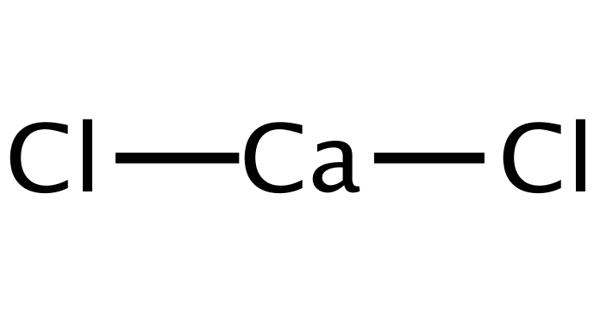Calcium chloride is one of the most versatile of the basic chemicals. It is an inorganic compound, a salt with the chemical formula CaCl2. It is a white to off-white solid. It is a white-colored crystalline solid at room temperature, and it is highly soluble in water. It sinks and mixes with water. It can be created by neutralizing hydrochloric acid with calcium hydroxide.
Calcium chloride is commonly encountered as a hydrated solid with generic formula CaCl2(H2O)x, where x = 0, 1, 2, 4, and 6. These compounds are mainly used for de-icing and dust control. Because the anhydrous salt is hygroscopic, it is used as a desiccant. It is commercially available under three different forms: two solid forms and one liquid.

Properties
Calcium chloride dissolves in water, producing chloride and the aquo complex [Ca(H2O)6]2+. In this way, these solutions are sources of “free” calcium and free chloride ions. This description is illustrated by the fact that these solutions react with phosphate sources to give a solid precipitate of calcium phosphate:
3 CaCl2 + 2 PO43- → Ca3(PO4)2 + 6 Cl–
Calcium chloride has a very high enthalpy change of solution, indicated by considerable temperature rise accompanying dissolution of the anhydrous salt in water. This property is the basis for its largest-scale application.
Molten calcium chloride can be electrolysed to give calcium metal and chlorine gas:
CaCl2 → Ca + Cl2
In much of the world, calcium chloride is derived from limestone as a by-product of the Solvay process, which follows the net reaction below:
2 NaCl + CaCO3 → Na2CO3 + CaCl2
Occurrence
Calcium dichloride is a calcium salt and an inorganic chloride. It occurs as the rare evaporite minerals sinjarite (dihydrate) and antarcticite (hexahydrate). Another natural hydrate known is ghiaraite – a tetrahydrate. The related minerals chlorocalcite (potassium calcium chloride, KCaCl3) and tachyhydrite (calcium magnesium chloride, Ca Mg2Cl6·12H2O) are also very rare. Although calcium chloride is highly soluble in water at ordinary temperatures, crystallization will occur under certain temperature and concentration conditions. So is true for rorisite, CaClF (calcium chloride fluoride). It has a role as a fertilizer.
Application
The properties and characteristics of CaCl2 make it useful in a large number of applications. It has several common applications such as brine for refrigeration plants, ice and dust control on roads, and in concrete.
It has been widely used as a preservative and firmness-increasing agent in the fruit and vegetable industry for whole, as well as for fresh-cut, produce.
Information Source: