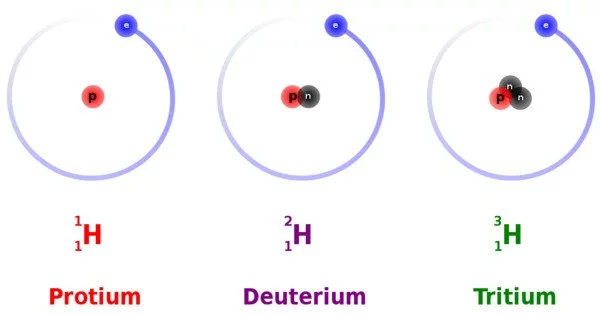
Deuterium is a stable isotope of hydrogen that has one proton and one neutron in its nucleus, as opposed to normal hydrogen (also called protium) which has only one proton in its nucleus. Deuterium is also sometimes referred to as “heavy hydrogen” because it has twice the atomic mass of normal hydrogen.
Deuterium is a hydrogen isotope, the first element. Deuterium consists of one proton and one neutron. Hydrogen does not have a neutron; instead, it has a proton. Tritium, another hydrogen isotope, has two neutrons. Deuterium’s chemical symbol is 2H, but the letter D is also frequently used.
On average, one deuterium isotope exists for every 6420 hydrogen atoms. Deuterium isotopes are found in molecules containing hydrogen, including, crucially, all forms of water – including water in our bodies. Every cubic metre of seawater contains 33 grams of deuterium, implying that the ocean contains tons of the isotope.
Deuterium is found in small quantities in nature, with about one atom of deuterium for every 6,400 atoms of protium. It is used in nuclear magnetic resonance (NMR) spectroscopy, where it is a useful probe for studying the structure of molecules. Deuterium is also used in nuclear fusion reactions as a fuel, because it can easily fuse with other deuterium atoms to form helium and release a large amount of energy.
The combination of two deuterium atoms and an oxygen atom is sometimes referred to as “heavy water.” Because deuterium has one more neutron in its nucleus, it is heavier than water (H2O). Heavy water is occasionally used in nuclear reactors. It is also used as a solvent for NMR spectroscopy. This is because, like normal water, it will dissolve the sample but will not be detected by the magnet in a 1H NMR.
The natural abundance of deuterium varies depending on the water source. Water is made up of hydrogen and oxygen atoms, so knowing the abundance of deuterium in a body of water can provide information about its history and origin. This is a crucial aspect of isotope hydrology. Deuterium has numerous other applications, including nutrition testing and use as a fuel in future fusion energy power plants.
Deuterium is also used in various applications in industry and research, such as in the production of heavy water, which is used as a moderator and coolant in nuclear reactors, and in the production of tritium, another isotope of hydrogen that is used in nuclear weapons and experimental fusion reactors.