Glycerol monostearate (GMS) is a monoglyceride that is commonly used as an emulsifier in foods. It is an effective emulsifier that is used in the baking industry and is available in small beads, flakes, or powders. GMS is a thickening agent and a stabilizer in addition to being an emulsifier. It takes the form of a hygroscopic white, odorless, and sweet-tasting flaky powder. Chemically, it is the stearic acid glycerol ester. It is classified as an emulsifier of mono- and diglycerides of fatty acids.
Properties
- Melting point: 78-81 °C
- Boiling point: 410.96°C (rough estimate)
- Density: 0.9700
- Solubility: Soluble in hot ethanol, ether, chloroform, hot acetone, mineral oil, and fixed oils.
- Form: Powder
- Color: Pure-white or cream-colored, wax-like solid
- Odor: faint odor

Preparation
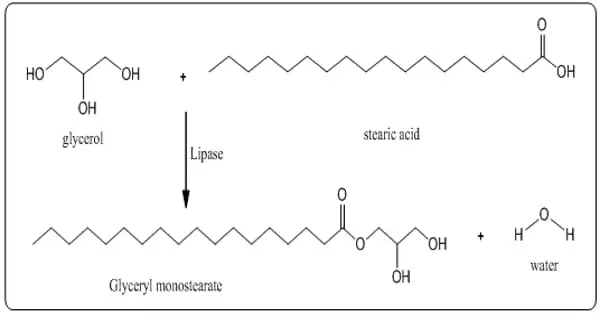
Glyceryl monostearate is made by glycerolysis of certain fats or oils derived from edible sources or by esterification of stearic acid derived from edible sources with glycerin. It has a waxy feel to it and a slight, mild fatty odor and taste. According to the USP, glyceryl monostearate contains at least 90% monoglycerides, primarily glyceryl monostearate and glyceryl monopalmitate.
Composition
GMS is a glycerol and stearic acid non-ionic ester. At 122°F (50°C), it is soluble in ethanol but immiscible in water. It is frequently composed of a mixture of mono, di, and triesters of fatty acids found in food oils and fats. They might have trace amounts of free fatty acids and glycerol.
Commercial Production
GMS is created by either heating oils/fats with excess glycerol or directly esterifying glycerol (from animal or plant sources) with stearic acid. The amount of monoester formed is proportional to the amount of glycerol and the reaction temperature range of 86-140°F (60-80°C). High vacuum distillation is used to further purify the product.
Food grade glycerol monostearate is commonly used as an emulsifier, as well as a stabilizer and humectant in bakery, confectionery, frozen desserts, oils, and fats. Aside from food, it can be used to make cosmetics, medicines, and plastics, among other things.
Structure, synthesis, and occurrence
There are three stereoisomers of glycerol monostearate, the enantiomeric pair of 1-glycerol monostearate and 2-glycerol monostearate. Because many of their properties are similar, these are frequently encountered as a mixture.
A glycerolysis reaction between triglycerides (from either vegetable or animal fats) and glycerol produces commercial material used in foods. It is found in very low concentrations in some seed oils. It is a stearic acid glycerol ester.
Glycerol monostearate is produced naturally in the body as a byproduct of fat breakdown by pancreatic lipase. It is found in fatty foods and occurs naturally in the body as a byproduct of fat breakdown.
Uses
GMS is a food additive that is used as a thickening, emulsifying, anticaking, and preservative agent; an emulsifying agent for oils, waxes, and solvents; a protective coating for hygroscopic powders; a pharmaceutical solidifier and control release agent; and a resin lubricant. It is used in baking to improve dough quality and to stabilize fat/protein emulsions. It’s also in cosmetics and hair care products.
GMS is commonly used in baking preparations to give the food “body.” It is partly responsible for the smooth texture of ice cream and whipped cream. It is sometimes used in bread as an antistaling agent.
GMS is a food additive that is used as a thickening, emulsifying, anti-caking, and preservative agent; an emulsifying agent for oils, waxes, and solvents; a protective coating for hygroscopic powders; a pharmaceutical solidifier and control release agent; and a resin lubricant. It’s also found in makeup and hair care products.
It can also be used as a plastic additive, where it acts as an antistatic and antifogging agent. This is a common practice in food packaging.