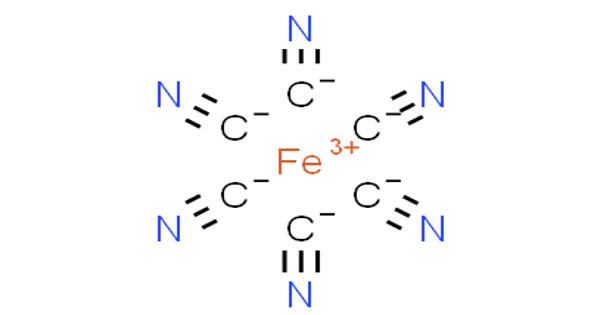
Ferricyanide is the anion [Fe(CN)6]3-. It is the complexion or any of various salts containing it, used in making blue pigments. It is also called hexacyanoferrate(III) and in rare, but systematic nomenclature, hexacyanidoferrate(III). It is a salt of ferricyanic acid containing the trivalent negative radical, in which the iron is trivalent. The most common salt of this anion is potassium ferricyanide, a red crystalline material that is used as an oxidant in organic chemistry. It is a complexion in which a central ferric iron atom is surrounded by six cyanide ions.
It is also formed by oxidizing bismuth trioxide suspended in caustic potash with chlorine, the pentoxide being formed simultaneously; oxidation and potassium ferricyanide simply gives the tetroxide (Hauser and Vanino, Zeit.
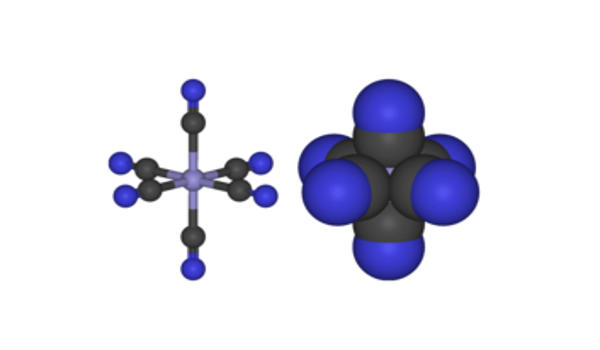
Properties
Ferricyanide addition is critical during membrane preparation. [Fe(CN)6]3- consists of a Fe3+ center bound in octahedral geometry to six cyanide ligands. The complex has Oh symmetry. This oxidant removes remaining electrons from the quinone pool and redox-active membrane proteins, thereby preventing oxidative damage of metal centers during purification under oxic conditions. The iron is low spin and easily reduced to the related ferrocyanide ion [Fe(CN)6]4-, which is a ferrous (Fe2+) derivative. This redox couple is reversible and entails no making or breaking of Fe–C bonds:
[Fe(CN)6]3- + e– ⇌ [Fe(CN)6]4-
This redox couple is a standard in electrochemistry.
Infra-red Spectra of the coal surface before and after exposure to ferricyanide showed that cyanide is strongly adsorbed on the coal surface. Compared to main group cyanides like potassium cyanide, ferricyanides are much less toxic because of the strong bond between the cyanide ion (CN− ) and the Fe3+. This indicates that ferricyanide can be added as an indicator of ferrous ion only after the iron solution has been removed from contact with the coal surface. They do react with mineral acids, however, to release highly toxic hydrogen cyanide gas.
Uses
Treatment of ferricyanide with iron(II) salts affords the brilliant, long-lasting pigment of Prussian blue, the traditional color of blueprints. The use of ferricyanide as a catholyte is unacceptable because of its toxic and hazardous characteristics. Employing air cathodes with expensive catalysts such as platinum to supplement their slow redox kinetics is also not permitted because of cost issues.
Information Source: