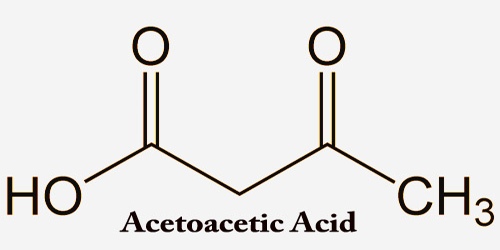
Acetoacetic acid (AcAc), also known as ‘diacetic acid’, is a weak organic acid that can be produced in the human liver under certain poor metabolism conditions that lead to excessive breakdown of fatty acids. It’s chemical formula CH3COCH2COOH, and acetoacetic acid is a weak acid. It’s the simplest beta-keto acid and it’s unstable like other members of the class. Acetoacetic acid is then partially converted by decarboxylation into acetone and excreted either in the urine or through respiration. The methyl and ethyl esters, which are quite stable, are industrially produced as precursors to dyes on a large scale.
Acetoacetic acid is a 3-oxo monocarboxylic acid that is butyric acid bearing a 3-oxo substituent. Acetoacetic acid exists as its conjugate base, acetoacetate, under typical physiological conditions. Acetoacetate is produced in the mitochondria of the liver from acetoacetyl coenzyme A (CoA). Acetoacetic acid plays a part as a metabolite. It is a ketone body and a 3-oxo fatty acid and it is a butyric acid derivative. The initial acetoacetate may originate in the beta-oxidation of fatty acid from the last cycle, or it may be synthesized from two molecules of acetyl CoA, which are catalyzed by thiolase.
Acetoacetic acid may be a conjugate acid of an acetoacetate; it’s a tautomer of a 3-hydroxy-3-butenoic acid. Its esters are produced analogously via a reaction between diketene and alcohols, and acetone body is often prepared by the hydrolysis of those species. In general, the ketone body is generated at 0 °C and employed in situ immediately. It decomposes at a moderate rate to acetone and carbon dioxide:
CH3C(O)CH2CO2H → CH3C(O)CH3 + CO2
Acetoacetate and beta-hydroxybutyrate are favored over glucose during the early postnatal period as substrates for the synthesis of phospholipids and sphingolipids, in line with brain growth and myelination requirements. The acid (Acetoacetic acid) form has a half-life of 140 minutes at 37 °C in water, whereas the basic form (the anion) has a half-life of 130 hours. That is, it reacts about 55 times more slowly.
Acetoacetic acid is additionally present within the metabolism of these undergoing starvation or prolonged workout as a part of gluconeogenesis. When ketone bodies are measured by way of urine concentration, acetone body, together with oxybutyric acid or acetone, is what’s detected. This acid may be employed in this reaction; diketene also reacts with alcohols and amines to the corresponding ketone body derivatives during a process called acetoacetylation.
In the lung, AcAc (Acetoacetic acid) serves better than glucose as a precursor for the synthesis of lung phospholipids. The synthesized lipids, particularly dipalmityl phosphatidylcholine, are incorporated into the surfactant, and thus have a potential role to play in providing adequate surfactant lipids to maintain lung function during the early days.
Information Sources: