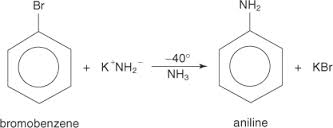
general objective of this article is to Analysis on Reactions of Aryl Halides. Aryl halides are relatively unreactive toward nucleophilic alternative reactions.. This lack of reactivity is a result of several factors. Steric hindrance caused by the benzene ring of the aryl halide prevents S N2 reactions. Likewise, phenyl cations are unstable, thus making S N1 reactions impossible. Additionally, the carbon‐halogen bond is shorter and as a consequence stronger in aryl halides than in alkyl halides.
Analysis on Reactions of Aryl Halides