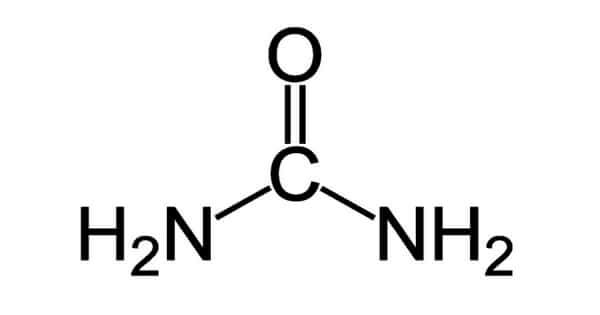
Urea, also known as carbamide, is a chemical compound that has the formula CO(NH2)2. It is also known as carbamide or carbonic acid diamide. A carbonyl (C=O) functional group connects two –NH2 groups in this amide. It is a soluble, weakly basic nitrogenous compound that is the main solid component of mammalian urine and an end product of protein decomposition. It is synthesized from carbon dioxide and ammonia and is used primarily in synthesis (as in resins and plastics) as well as fertilizers and animal feed.
In 1828, Friedrich Wöhler discovered that urea can be synthesized from inorganic starting materials, which was a significant conceptual breakthrough in chemistry. It demonstrated for the first time that a substance previously known only as a byproduct of life could be synthesized in the laboratory without the use of biological starting materials, thus contradicting the widely held vitalism doctrine, which stated that only living things could produce the chemicals of life.
Properties
Urea is a nitrogenous compound with an osmotic diuretic activity that contains a carbonyl group attached to two amine groups. It is a colorless, crystalline substance that melts at 132.7 degrees Celsius (271 degrees Fahrenheit) and decomposes before boiling. It is neither acidic nor alkaline when dissolved in water.
Urea is the final end product of protein metabolism and is formed in the liver via the urea cycle from ammonia. Urea raises blood plasma osmolality, resulting in an increased flow of water from tissues such as the brain, cerebrospinal fluid, and eye into interstitial fluid and plasma, lowering the pressure in those tissues and increasing urine outflow.

Chemical Symmetry
Urea is a carbonyl group with two amine groups that are C-bound. It is a human metabolite, a Daphnia Magna metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a mouse metabolite, and a fertilizer. It is a monocarboxylic acid amide with one carbon. It is made from carbonic acid. It is a carbamimidic acid tautomer.
Urea is the main nitrogen-containing substance in mammalian urine and plays an important role in the metabolism of nitrogen-containing compounds by animals. It is a colorless, odorless solid that is highly soluble in water and virtually non-toxic (LD50 for rats is 15 g/kg). It is used by the body in a variety of processes, the most notable of which is nitrogen excretion. In the urea cycle, the liver creates it by combining two ammonia molecules (NH3) with a carbon dioxide (CO2) molecule. Urea is a nitrogen (N) source that is widely used in fertilizers and is an important raw material in the chemical industry.
Uses
Urea is useful as a fertilizer and feed supplement, as well as a starting material in the production of plastics and drugs. Urea is the primary nitrogenous byproduct of protein breakdown in all mammals and some fishes. The substance is found not only in all mammals’ urine, but also in their blood, bile, milk, and perspiration.