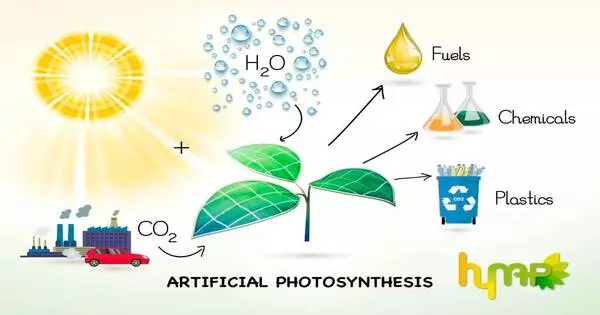
Artificial photosynthesis is a chemical process that mimics the natural process of photosynthesis by converting sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term artificial photosynthesis is commonly used to refer to any scheme for capturing and storing energy from sunlight in the chemical bonds of a fuel (solar fuel). Photocatalytic water splitting, which converts water into hydrogen and oxygen, is a major research topic in artificial photosynthesis. Another process being studied that mimics natural carbon fixation is light-driven carbon dioxide reduction.
The goal of artificial photosynthesis is to develop systems that efficiently capture and convert solar energy into chemical fuels such as hydrogen or hydrocarbons, which can then be stored and used as a clean and renewable energy source. This technology has the potential to address some of the world’s most pressing energy and environmental issues, such as lowering greenhouse gas emissions and providing renewable energy sources.
Artificial photosynthesis is a field of study that seeks to mimic the natural photosynthesis process that occurs in plants and some microorganisms. Natural photosynthesis is the biological process by which green plants, algae, and certain bacteria convert sunlight, water, and carbon dioxide into glucose and oxygen, with the energy from the sun fueling the chemical reactions.
Key components of artificial photosynthesis typically include:
- Light Absorption: Just like in natural photosynthesis, artificial photosynthesis systems need light-absorbing materials to capture solar energy. These materials are often semiconductors or light-sensitive molecules.
- Water Splitting: In the first step, water molecules are split into oxygen and protons (hydrogen ions). This process is called water oxidation and requires a source of energy, which in artificial photosynthesis, comes from the absorbed sunlight.
- Carbon Dioxide Reduction: The second step involves the reduction of carbon dioxide using the protons and electrons generated during water splitting. This produces hydrocarbons or other valuable chemicals, depending on the desired end product.
- Catalysts: Catalysts are used to facilitate the chemical reactions involved in both water splitting and carbon dioxide reduction, making these processes more efficient.
- Separation and Storage: The final step is to separate and store the produced fuels or chemicals for later use. This step can involve various technologies, such as fuel cells or chemical storage systems.
Artificial photosynthesis holds great promise for generating sustainable energy and combating climate change, but significant challenges remain, such as developing materials that are efficient and durable enough for practical applications and scaling up these technologies to meet global energy demands. Nonetheless, it is an exciting and rapidly evolving field of study that has the potential to change the way we produce and use energy in the future.
The design and assembly of devices for the direct production of solar fuels, photoelectrochemistry and its application in fuel cells, and the engineering of enzymes and photoautotrophic microorganisms for microbial biofuel and biohydrogen production from sunlight are all part of this research.