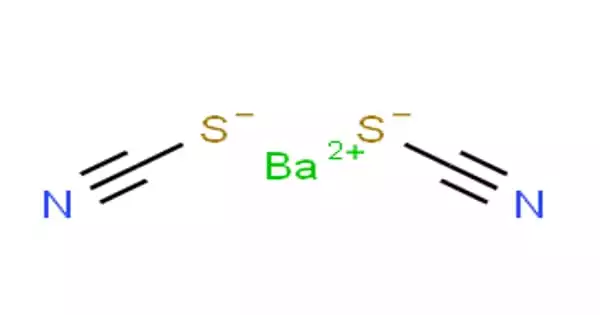
Barium thiocyanate is a colorless, water-soluble salt that is very hygroscopic. The barium salt of thiocyanic acid, Ba(SCN)2, used in photography and in dyeing. It is highly toxic to ingestion and irritates the skin. It is also soluble in most alcohols and insoluble in simple alkanes.
Thiocyanate is one of the most important spectrophotometric reagents. The availability of the reagent and the simplicity of thiocyanate methods are responsible for its great popularity in analytical laboratories.
Properties
It is a white, needle-shaped crystal that are used in dyeing textiles. It is also an ingredient in some photographic solutions.
- Molecular Weight: 253.5
- Color & Form: White solid
- Specific Gravity: 2.286
- Solubility in water: Soluble
- Density: 3.5 g/ml

Uses
Barium thiocyanate is used in dyeing textiles and is an ingredient in some photographic solutions. But because of its toxicity, it has limited uses.
Preparation
Barium thiocyanate is prepared by dissolving barium metal or barium nitrate in a solution of thiocyanic acid. It is used in dyeing, photography, in preparation of thiocyanates of other metals, and as a dispersing agent for cellulose.
Thiocyanate is separated from cyanide by distilling off HCN from a weakly acid medium. With bromine and chloramine-T, thiocyanate is converted into cyanogen bromide and cyanogen chloride, respectively, and determined as a polymethine dye by the benzidine-pyridine method or pyridine–barbituric acid method
Risks
- Highly toxic by ingestion.
- Contact with skin and membranes may cause irritation.