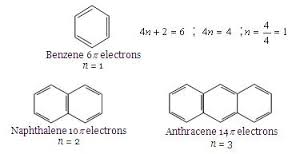
Basic purpose of this article is to Define and Discuss on Hückel’s Rule. In 1931, Erich Hückel postulated that monocyclic planar compounds that contained carbon atoms having unhybridized atomic p orbitals would contain a closed bond shell connected with delocalized π electrons if the volume of π electrons in this molecule fit a value of 4n+3 where n equaled just about any whole number. Because any closed bond shell connected with π electrons defines a great aromatic system, you will use Hückel’s Rule to predict the aromaticity of an compound.
Define and Discuss on Hückel’s Rule