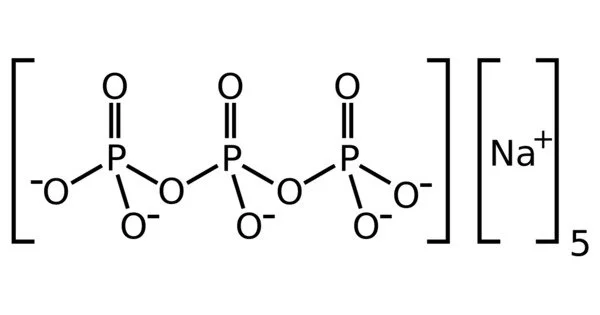
Sodium triphosphate (STP) is an inorganic compound with the formula Na5P3O10. It is the sodium salt of the polyphosphate penta-anion, which is the conjugate base of triphosphoric acid. It is widely used as a component in many household and industrial products, particularly detergents. The widespread use of eutrophication causes environmental problems.
It is commonly used as a food additive, particularly in the meat processing industry, as a preservative and to increase the water-holding capacity of meat products. It is also used in detergents as a builder, helping to soften hard water and improve the effectiveness of the cleaning agents.
Properties
It is an inorganic compound and a member of the polyphosphate family, which are salts of polymeric oxyanions formed from tetrahedral PO4 units linked by sharing oxygen atoms. It is a white, crystalline powder that is highly soluble in water.
- Chemical formula: Na5P3O10
- Molar mass: 367.864 g/mol
- Appearance: white powder
- Density: 2.52 g/cm3
- Melting point: 622 °C (1,152 °F; 895 K)
- Solubility in water: 14.5 g/100 mL (25 °C)

Preparation
Sodium tripolyphosphate is produced by heating a stoichiometric mixture of disodium phosphate, Na2HPO4, and monosodium phosphate, NaH2PO4, under carefully controlled conditions.
2 Na2HPO4 + NaH2PO4 → Na5P3O10 + 2 H2O
In this way, approximately 2 million tons are produced annually.
STPP is a colourless salt, which exists both in anhydrous form and as a hexahydrate. The anion can be described as the pentanionic chain [O3POP(O)2OPO3]5−. Many related di-, tri-, and polyphosphates are known including the cyclic triphosphate (e.g. sodium trimetaphosphate). It binds strongly to metal cations as both a bidentate and tridentate chelating agent.
Application
One of the main uses of sodium triphosphate is as a builder in detergents and soaps. It helps to soften water by binding to calcium and magnesium ions, which can interfere with the effectiveness of cleaning agents. It also helps to prevent soil and dirt from re-depositing onto fabrics during the wash cycle.
Sodium triphosphate has also been used in a variety of industrial applications, such as in ceramic production, petroleum refining, and water treatment. However, its use has been restricted or banned in some countries due to concerns about its environmental impact and potential health hazards.
Health effects
A high serum phosphate concentration has been linked to cardiovascular events and mortality. While organic forms of phosphate are present in the body and food, inorganic forms of phosphate, such as sodium triphosphate, are easily absorbed and can result in elevated phosphate levels in serum. Because polyphosphate anions are mildly alkaline, they are moderately irritating to the skin and mucous membranes.
It has been found to contribute to eutrophication, or the excessive growth of algae in bodies of water when it is released into the environment. In addition, high levels of sodium triphosphate in food products have been linked to potential health risks, such as kidney damage and high blood pressure.
Environmental effects
STPP is not significantly removed by waste-water treatment due to its high water solubility. STPP hydrolyzes to phosphate, which enters the natural phosphorus cycle. Phosphorus-containing detergents contribute to the eutrophication of many freshwater ecosystems.