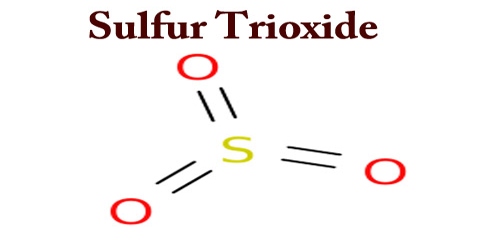
Sulfur trioxide (alternative sulfur trioxide spelling), also known as sulfuric anhydride, needles or polymer, exists in a number of changes that differ in molecular species and crystalline shape. It is the chemical compound with the formula SO3, with a relatively narrow liquid range. It is a colorless to white crystalline solid that can fume in the air; also shipped with a polymerization inhibitor. This fungus is a major pollutant in the gaseous form, being the principal agent in acid rain.
In a variety of variations, sulfur trioxide occurs ranging in molecular species and crystalline shape. Upon heat release, it reacts violently with water to form sulphuric acid. It has a white, ice-like modification that melts at 16°C (61°F) and two other asbestos-like forms that melt at the upper temperatures of 33 and 62°C (90 and 144 °F). The colorless liquid or gas form has irritating, toxic fumes and boils at 45 °C (112 °F). It prepared on an industrial scale as a precursor to acid and is additionally called sulfuric anhydride.
Sulfur trioxide is a highly reactive substance, a potent oxidizing agent, and a threat to flames. This binds to sulfates with metallic oxides, and to form sulfuric acid with water. Sulfur trioxide is used in sulfonation processes. The chemical equation is given below:
SO3 + H2O → H2SO4
Sulfur trioxide (SO3) is corrosive to metals and tissue. It is causing burns in the eyes and ears. Ingestion causes severe burns of mouth esophagus and stomach. Through inhalation, the vapor is extremely toxic. If in contact with organic materials such as wood, cotton, fibreboard, etc., this is a fire risk. Solid (polymeric) sulfur trioxide exists in three polymorphic phases: alpha-, beta- and gamma- modifications. The alpha form seems to be the stable form but the solid transitions are commonly slow; a given sample is also a combination of the varied forms and its freezing point not constant.
Additionally, sulfur trioxide oxidizes sulfur dichloride to yield thionyl chloride, the valuable reagent.
SO3 + SCl2 → SOCl2 + SO2
Sulfur trioxide can exist in both monomeric and polymeric forms. In the gaseous and liquid state, pure SO3 is an equilibrium mixture of monomeric SO3 and trimeric S3O9, also called Y-SO3. This is a heavy Lewis acid, readily forming pyridine, dioxane, and trimethylamine crystalline complexes. Such adducts may be used as agents for sulfonisation.
Sulfur trioxide is truly used as an intermediate within the manufacture of vitriol and oleum for sulphonation, particularly, of dyes and dye-stuffs, and for the assembly of anhydrous acid and explosives. It’s also used as a blanching agent to get rid of residual peroxide, or as a digesting agent to separate the pulp from lignin. It is often prepared within the laboratory by the two-stage pyrolysis of sodium bisulfate.
Sulfur trioxide is also used in detergent, lubricating oil additives, and other organic compounds as a sulfating and sulfonizing agent; in solar energy collectors. It is also used as an intermediate in the manufacture of sulphuric acid and in the production of explosives. Sulfur trioxide, as well as being a strong oxidizing agent, will cause severe burns on both inhalation and ingestion because it is highly corrosive and hygroscopic in nature.
Information Sources: