A group of scientists has created a water-activated disposable paper battery. According to the researchers, it could be used to power a wide range of low-power, single-use disposable electronics, such as smart labels for tracking objects, environmental sensors, and medical diagnostic devices, while minimizing their environmental impact.
Gustav Nyström and his team designed the battery, which consists of at least one cell measuring one centimeter squared and consisting of three inks printed onto a rectangular strip of paper. The strip of paper is sprinkled with salt, in this case simply sodium chloride or table salt, and one of its shorter ends has been dipped in wax.
An ink containing graphite flakes, which acts as the positive end of the battery (the cathode), is printed onto one of the flat sides of the paper, while an ink containing zinc powder, which acts as the negative end of the battery (the anode), is printed onto the reverse side of the paper. On top of the other two inks, a third ink containing graphite flakes and carbon black is printed on both sides of the paper. This ink forms the current collectors that connect the positive and negative ends of the battery to two wires located at the wax-dipped end of the paper.
What makes our new battery unique is that, unlike many metal air batteries that use a metal foil that is gradually consumed as the battery depletes, our design allows us to add only the amount of zinc to the ink that is actually needed for the specific application.
Gustav Nyström
When a small amount of water is added, the salts in the paper dissolve, and charged ions are released, resulting in an ionically conductive electrolyte. These ions activate the battery by dispersing through the paper and oxidizing zinc in the ink at the anode, releasing electrons. By closing the (external) circuit, electrons can be transferred from the zinc-containing anode to the graphite cathode, where they are transferred to – and thus reduce – oxygen from ambient air via the graphite- and carbon black-containing ink, wires, and device. As a result of these redox reactions (reduction and oxidation), an electrical current is generated that can be used to power an external electrical device.
Proof of concept: a sustainable energy source for low-power electronics
To demonstrate the ability of their battery to run low-power electronics, Nyström’s team combined two cells into one battery to increase the operating voltage and used it to power an alarm clock with a liquid crystal display. Analysis of the performance of a one-cell battery revealed that after two drops of water were added, the battery activated within 20 seconds and, when not connected to an energy-consuming device, reached a stable voltage of 1.2 volts. The voltage of a standard AA alkaline battery is 1.5 volts.
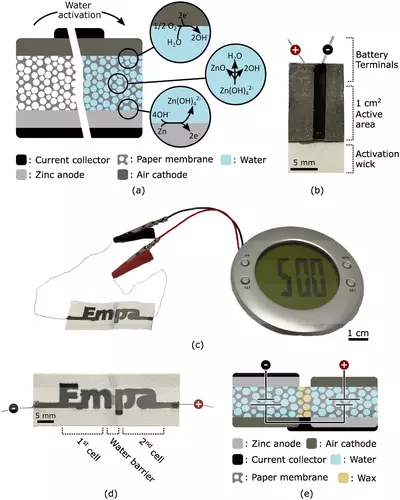
Due to the drying of the paper, the performance of the one-cell battery decreased significantly after one hour. The battery maintained a stable operating voltage of 0.5 volts for more than an hour after the researchers added two extra drops of water.
The biodegradability of paper and zinc, according to the researchers, could allow their battery to reduce the environmental impact of disposable, low-power electronics. “What makes our new battery unique is that, unlike many metal air batteries that use a metal foil that is gradually consumed as the battery depletes,” says Nyström, “our design allows us to add only the amount of zinc to the ink that is actually needed for the specific application.” Metal foils were more difficult to control and not always fully consumed leading to a waste of materials. So the more zinc the ink contains, the longer the battery is able to operate.
A more critical point of the battery’s current design with water activation, Nyström adds, is the time it takes for the battery to dry out. “But I am sure this can be engineered differently to get around this problem.” For environmental sensing applications at a certain humidity or in wet environments, however, the drying of the paper would not be an issue.
Two complementary technologies
Nyström’s team had previously developed a degradable super capacitor made of paper that could be charged and discharged thousands of times without losing efficiency. Supercapacitors have an energy density that is approximately ten times lower than batteries of the same weight while having a power density that is approximately ten to one hundred times greater.
As a result, supercapacitors can charge and discharge much faster. They are also more resistant to charge and discharge cycles. “As a result, the two devices are actually complementary,” Nyström says. The idea behind the new water-activated battery was to be able to create devices that are fully charged but only release this energy when a stimulus, in this case a drop of water, was triggered.