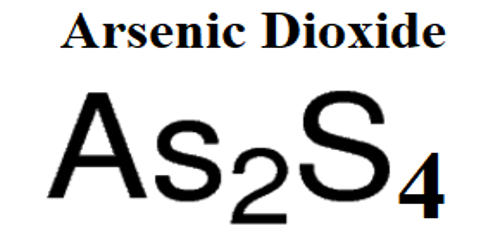Arsenic is the third member of the nitrogen family of elements. In the majority of arsenic compounds, the arsenic atom is in the tetrahedral valence state. The arsenic dioxide is an inorganic compound with the chemical formula As2O4, containing As(III) and As(V), AsIIIAsVO4. It appears as a white crystalline solid. Common arsenic oxide minerals include arsenates or As5 + species such as scorodite, arseniosiderite, and pharmacosiderite and aresnites or As3 + species such as claudetite (As2O3) and arsenolite (As2O3). It is stable in dry air, but the surface oxidizes slowly in moist air to give a bronze tarnish and finally a black covering to the element.
Arsenic is a recognized reproductive toxicant in humans that induces malformations and that has been identified as a pollutant in the air, soil, and water. The arsenic dioxide is common in mine wastes and mine-drainage settings, mainly because of the common and widespread occurrence of arsenopyrite and arsenian pyrite as gangue minerals.
Properties
- Formula: AsO2
- Molecular weight: 106.9204
- IUPAC Standard InChI: 1S/AsO2/c2-1-3
- IUPAC Standard InChIKey: LZYIDMKXGSDQMT-UHFFFAOYSA-N
- CAS Registry Number: 12255-12-8
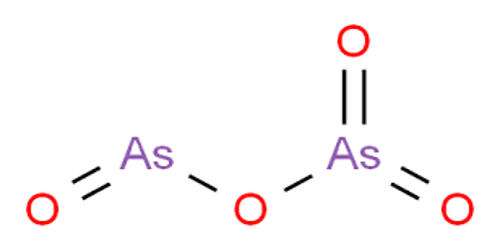
Fig: Arsenic Dioxide Structure
Synthesis
It can be synthesized in an autoclave via the following reaction: 2 As2O3 + O2 → 2 As2O4
Arsenic reacts under controlled conditions with the halogens fluorine, F2, chlorine, Cl2, bromine, Br2, and iodine, I2, to form the respective trihalides arsenic(III) fluoride, AsF3, arsenic(III) chloride, AsCl3, arsenic(III) bromide, AsBr3, and arsenic(III) iodide, AsI3.
Structure
It adopts a layer structure, and the coordination geometry of As(III) is a triangular pyramid while As(V) is tetrahedral. Thermal decomposition of the pentoxide converts it to the trioxide with concurrent loss of oxygen. The pentoxide, in contrast with the trioxide, is very soluble in water; 630 g of arsenic pentoxide dissolve in 100 g of water.
Uses
Arsenic compounds must be considered extremely poisonous. Arsenic compounds are used in fertilizers, herbicides, preparation of wood preservative (CCA), fireworks, insecticides, and pharmaceuticals.
Safety
One of the simplest arsenic compounds is the trihydride, the highly toxic, flammable, pyrophoric arsine (AsH3). This compound is generally regarded as stable since at room temperature it decomposes only slowly. Arsenic trioxide is the primary product of arsenic smelters. This oxide has direct applications in industry—e.g., as a glass decolorizing agent. Other commercially useful organic and inorganic arsenic derivatives are prepared from it.