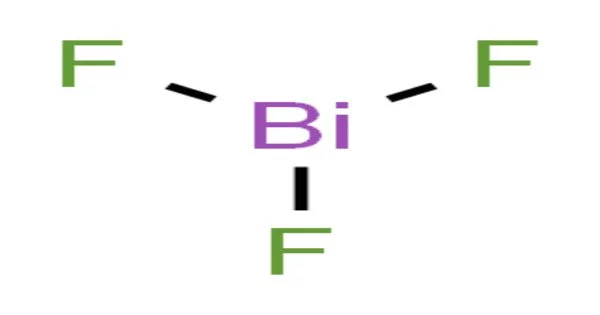
Bismuth(III) fluoride or bismuth trifluoride is a chemical compound of bismuth and fluorine. The chemical formula is BiF3. It is a grey-white powder melting at 649 °C. It is a white powder, golden yellow crystals, greyish-black crystals, are also known.
It occurs in nature as the rare mineral gananite.
Synthesis
Bismuth trifluoride can be prepared by reacting bismuth(III) oxide with hydrofluoric acid:
Bi2O3 + 6 HF → 2 BiF3 + 3 H2O
Properties
- Chemical formula: BiF3
- Molar mass: 265.97550 g/mol
- Appearance: grey-white powder
- Density: 5.32 g cm−3
- Melting point: 649˚C
- Solubility in water: Insoluble in water
- Magnetic susceptibility (χ): -61.0·10−6 cm3/mol

Structure
The cubic crystalline structure of α-BiF3 (Pearson symbol cF16, space group Fm-3m, No. 225). BiF3 is the prototype for the D03 structure, which is used by a number of intermetallics such as Mg3Pr, Cu3Sb, Fe3Si, and AlFe3, as well as the hydride LaH3.0. The unit cell is face-centered cubic, with Bi at the face centers and vertices and F at the octahedral (mid-edges, center) and tetrahedral (centers of the 8 subcubes) sites – thus the primitive cell contains 4 Bi and 12 F.
Alternatively, with the unit cell shifted (1/4,1/4,1/4), the description can be of a fcc cell with F filling the face, edge, corner, and centers, and F filling half (4 of) the octant centers and Bi filling the other half (each octant type tetrahedrally arranged). The BiF3 cell has an edge length of 0.5853 nm. β-BiF3 has the YF3 structure, with a distorted 9 coordination, tricapped trigonal prism. This structure is generally thought to be ionic, in contrast to fluorides of the lighter members of group 15, phosphorus trifluoride, PF3, arsenic trifluoride, AsF3, and antimony trifluoride, SbF3, all of which contain MX3 molecular units in the solid.
Reactions
Water has no effect on BiF3, and it is almost insoluble. It does not readily form complexes, but the following are known: BiF3.3HF and BiF4– in NH4BiF4. Water hydrolyzes the addition compound H3BiF6, forming BiOF.
Uses
BiF3 has attracted interest in research as a potential electrode material for lithium batteries and as a luminescence host material for lanthanum-doped phosphors.