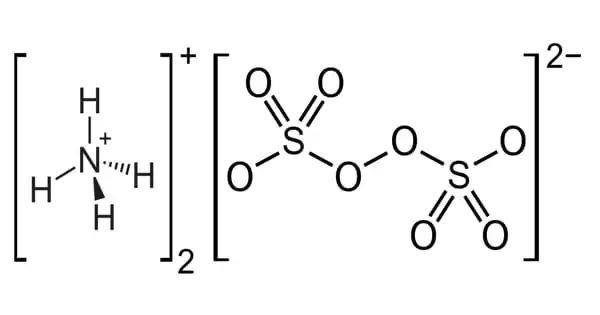
Ammonium persulfate (APS) is an inorganic chemical having the formula (NH4)2S2O8. It is a white crystalline substance with no odor. It is a colorless (white) salt that is extremely soluble in water, far more so than the related potassium salt. It is easily soluble in water and is a powerful oxidizing agent. It is a strong oxidizing agent used in polymer chemistry, as an etchant, and as a cleaning and bleaching agent. The dissolving of salt in water is an endothermic reaction. It is used in polymer chemistry as a bleaching agent, cleaning agent, and etchant.
It has the appearance of a white crystalline solid. It is a powerful oxidizing agent. Although it does not burn easily, it has the potential to produce spontaneous combustion of organic compounds. It is used as a bleaching agent as well as a food preservative.
Properties
It is a colorless salt that is substantially more soluble in water than the comparable potassium salt. It is a strong oxidizing agent used in polymer chemistry, cleaning and bleaching, and as an etchant. Ammonium peroxydisulfate is sometimes known as diammonium persulfate or diammonium peroxydisulfate.
- Molecular weight: 228.18 g/mol
- Density: 1.98 g/dm3
- Flashpoint: Non-flammable
- Melting Point: 120 °C

Preparation
Ammonium persulfate is made by electrolysis at a high current density of a cold concentrated solution of either ammonium sulfate or ammonium bisulfate in sulfuric acid. Hugh Marshall was the first to describe the procedure.
It is made by electrolyzing a cold concentrated solution of either ammonium sulfate or ammonium bisulfate in the presence of sulfuric acid at a very high current density.
Uses
As an oxidizing agent and a source of radicals, APS finds many commercial applications.
Salts of sulfate are mainly used as radical initiators in the polymerization of certain alkenes. Commercially important polymers prepared using persulfates include styrene-butadiene rubber and polytetrafluoroethylene. In solution, the dianion dissociates to give radicals:
[O3SO–OSO3]2− ⇌ 2 [SO4]•−
The sulfate radical adds to the alkene to give a sulfate ester radical. It is also used along with tetramethylethylenediamine to catalyze the polymerization of acrylamide in making a polyacrylamide gel, hence being important for SDS-PAGE and western blot.
It is used to etch copper on printed circuit boards as an alternative to ferric chloride solution, demonstrating its intense oxidizing characteristics. This property has been known for many years. In 1908, John William Turrentine etched copper with a dilute ammonium persulfate solution. Turrentine weighed copper spirals before immersing them in an ammonium persulfate solution for an hour. The spirals were weighed again after an hour, and the amount of copper dissolved by ammonium persulfate was recorded. This experiment was repeated with other metals such as nickel, cadmium, and iron, with comparable results. The oxidation equation is thus: S2O82- (aq) + 2 e– → 2 SO42- (aq).
Ammonium persulfate is a standard ingredient in hair bleach.
Persulfates are used as oxidants in organic chemistry. For example, in the Minisci reaction.
It is used across several industries in the following ways:
- It is used in the printed circuit boards.
- It is used for photography.
- It is used as an additive for preserving the food.
- It is used as an oxidising agent.
- It is used to wash the infected yeast.
- It is used as a depolarizer in batteries.
Safety
Contact with ammonium persulfate-containing airborne dust may cause irritation to the eyes, nose, throat, lungs, and skin. Breathing difficulties may occur if exposed to excessive levels of dust.
Persulfate salts have been identified as a key source of asthmatic consequences. Furthermore, exposure to ammonium persulfate has been linked to asthmatic symptoms among hairdressers and receptionists working in the business. These asthmatic effects are thought to be caused by the oxidation of cysteine and methionine residues.