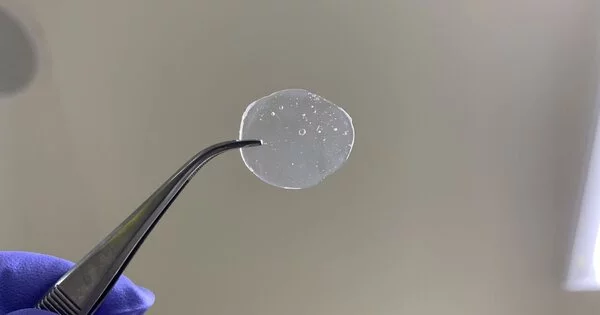
Solid hydrogen is the solid phase of hydrogen, which is a chemical element with the symbol H and atomic number 1. It is the solid state of the element hydrogen, achieved by decreasing the temperature below hydrogen’s melting point of 14.01 K (−259.14 °C; −434.45 °F). At standard temperature and pressure, hydrogen is a colorless, odorless, tasteless, non-toxic, and highly flammable diatomic gas with the molecular formula H2. It has a density of 0.086 g/cm3 making it one of the lowest-density solids.
Solid hydrogen is formed when gaseous hydrogen is cooled below its boiling point of -252.87°C (-423.17°F) and put under high pressure. The resulting solid is a very light, crystalline substance that is transparent and nearly colorless. Solid hydrogen is also known as hydrogen ice or molecular hydrogen, and it has been the subject of intense scientific research due to its unique properties and potential applications.
Molecular solid hydrogen
At low temperatures and pressures up to 400 GPa, hydrogen condenses into a series of solid phases composed of discrete H2 molecules. At low temperatures and pressures, phase I is composed of a hexagonal close-packed array of freely rotating H2 molecules. A transition to Phase II occurs at up to 110 GPa when pressure is increased at low temperatures. Phase II is a broken-symmetry structure in which the H2 molecules cannot freely rotate. When the pressure is increased further at low temperature, a Phase III is reached at around 160 GPa. When the temperature rises to a few hundred degrees Celsius and the pressure rises above 220 GPa, a transition to Phase IV occurs.
Solid hydrogen is a highly sought-after material in the fields of condensed matter physics, materials science, and engineering, as it exhibits a range of interesting properties such as high thermal conductivity, high optical transparency, and superconductivity at very low temperatures. It is also being studied as a potential fuel source for rocket propulsion, as it has a very high energy density.
Application
Some potential applications of solid hydrogen include its use as a rocket fuel, as a high-energy storage medium for batteries, and as a superconductor at low temperatures. However, the production of solid hydrogen is extremely challenging and requires sophisticated equipment and techniques, which has limited its practical applications so far.
However, solid hydrogen is difficult to work with due to its extremely low temperatures and high pressure requirements, which can make it unstable and difficult to handle. The process of producing and storing solid hydrogen also presents significant technical challenges, and there are safety concerns associated with its use.