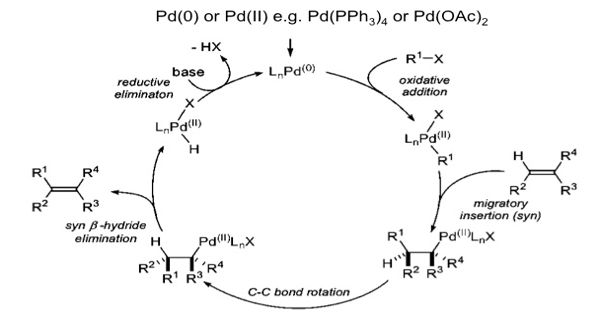
The Heck reaction is the palladium-catalyzed cross-coupling reaction between alkenes, and aryl or vinyl halides (or triflates) to afford substituted alkenes. It is an organic chemical reaction in which an unsaturated halide reacts with an alkene in the presence of a palladium catalyst and a base. It is also called the Mizoroki–Heck reaction which is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a substituted alkene. The palladium-catalyzed C-C coupling between aryl halides or vinyl halides and activated alkenes in the presence of a base is referred to as the “Heck Reaction”.
The Heck reactions are the reactions of aryl halides with alkenes in the presence of palladium catalysts and are the most industrially utilized reactions. It is considered to be one of the more useful strategies in organic synthesis for the construction of carbon-carbon bonds.
The Heck reaction is a famous chemical reaction discovered by Mizoroki and Heck in 1972 through independent research. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. It is described as a vinylation or arylation of olefins where a large variety of olefins can be used, like derivatives of acrylates, styrenes, or intramolecular double bonds.
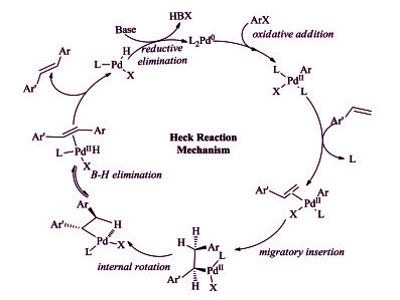
The Heck reaction proceeds under relatively mild conditions tolerate a variety of functional groups such as aldehydes, amines, esters, and nitro groups, and often affords high yields of desired products. The reaction yields a substituted alkene and is considered to be a coupling reaction. It remains one of the most widely used Pd-catalyzed methodologies in synthesis and catalyst discovery and optimization. One of the major limitations of the Heck reaction is the precipitation of Pd metal, which results in catalyst deactivation.
It is a useful carbon-carbon bond-forming reaction with synthetic importance. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The reaction proceeds in the presence of a base and it is highly stereoselective in nature. The Heck reaction is a way to substitute alkenes.
Information Source: