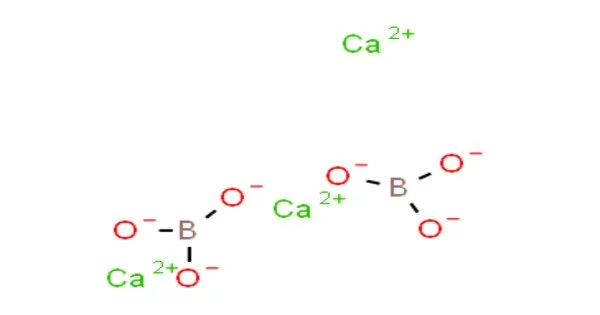
Calcium borate, also known as calcium tetraborate or CaB4O7, is a chemical compound composed of calcium, boron, and oxygen. It has a white crystalline powder appearance and is used in a variety of applications. The compound has a high melting point of around 1,200 degrees Celsius and is relatively stable at high temperatures.
In terms of its chemical properties, calcium borate is relatively stable at high temperatures and is insoluble in water. It can be produced by reacting borax (sodium borate) with calcium chloride or calcium oxide.
Properties
- Physical appearance: It is a white to greyish-white powder or crystalline solid.
- Melting point: The melting point is approximately 1200°C.
- Solubility: It is insoluble in water but soluble in acids.
- Density: The density is approximately 2.46 g/cm³.
- Stability: It is stable at room temperature and does not decompose easily.
- Thermal conductivity: It has a low thermal conductivity, which makes it useful as an insulating material.
- Chemical properties: It is a weekly basic compound and reacts with acids to form borates.
- Electrical conductivity: It is an insulator and does not conduct electricity.
- Flame retardant properties: It has flame retardant properties and is used in the manufacture of fire-resistant materials.
Occurrences
Calcium borate is relatively insoluble in water, but it can dissolve in acids and forms basic solutions. It is also found in nature as a mineral called colemanite, which is a source of both boron and calcium. The mineral is typically found in sedimentary rock formations and is primarily mined in Turkey and the United States.
Application
Calcium borate is commonly used as a flame retardant in plastics, coatings, and textiles. It is also used as a flux in metallurgy and as a source of boron in agriculture. Additionally, it is used in the manufacture of glass and ceramics.
One of the main uses of calcium borate is as a flame retardant in various materials, including plastics, textiles, and wood. It is also used as a flux in welding and soldering, as well as in the production of glass and ceramics.
Calcium borate can also be found in some cosmetics and personal care products, such as toothpaste and deodorants, where it functions as an antiseptic and astringent.