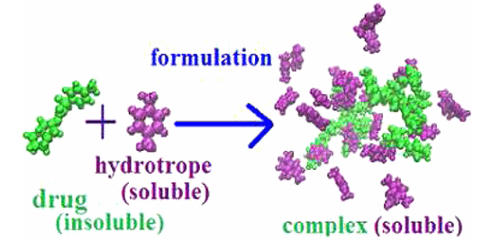
A hydrotrope is a compound that solubilizes hydrophobic compounds in aqueous solutions by means other than micellar solubilization. They are water-soluble, surface-active compounds that can significantly affect the solubility of poorly soluble drugs, most likely due to the formation of organized assemblies within the solution. Hydrotropes are small molecules that solubilize hydrophobic molecules in aqueous solutions. Typically, hydrotropes consist of a hydrophilic part and a hydrophobic part (similar to surfactants), but the hydrophobic part is generally too small to cause spontaneous self-aggregation. Typically, hydrotropes are amphiphilic molecules and differ from classical surfactants in that they have low cooperativity of aggregation and work at molar concentrations
A hydrotrope is an organic salt compound that improves the ability of water to dissolve other molecules by solubilizing hydrophobic compounds. Some hydrotropes may aggregate in a stepwise manner, although many show no signs of self-aggregation even at high concentrations. Hydrotropes do not have a critical concentration above which self-aggregation spontaneously starts to occur. Instead, some hydrotropes aggregate in a step-wise self-aggregation process, gradually increasing aggregation size. Hydrotropes, unlike emulsifiers, do not have a critical concentration above which self-aggregation “suddenly” starts to occur. However, many hydrotropes do not seem to self-aggregate at all, unless a solubilizate has been added. Examples of hydrotropes include urea, tosylate, cumenesulfonate, and xylenesulfonate. Instead, some hydrotropes aggregate in a step-wise self-aggregation process, gradually increasing in size.
The term hydrotropy was originally put forward by Carl Neuberg to describe the increase in the solubility of a solute by the addition of fairly high concentrations of alkali metal salts of various organic acids. However, the term has been used in the literature to designate non-micelle-forming substances, either liquids or solids, organic or inorganic, capable of solubilizing insoluble compounds. Hydrotropes (such as toluene sulphonate) are also used to enhance the solubility of organic materials in the water base.
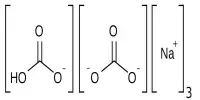
Hydrotropes may either increase or decrease the solubility of a solute in a given solvent. Those that increase solubility and are said to salt in the solute and those that decrease solubility are said to salt out the solute. The chemical structure of the conventional Neuberg’s hydrotropic salts (proto-type, sodium benzoate) consists generally of two essential parts, an anionic group, and a hydrophobic aromatic ring or ring system. The anionic group is involved in bringing about high aqueous solubility, which is a prerequisite for a hydrotropic substance. The type of anion or metal ion appeared to have a minor effect on the phenomenon. On the other hand, the planarity of the hydrophobic part has been emphasized as an important factor in the mechanism of hydrotropic solubilization.