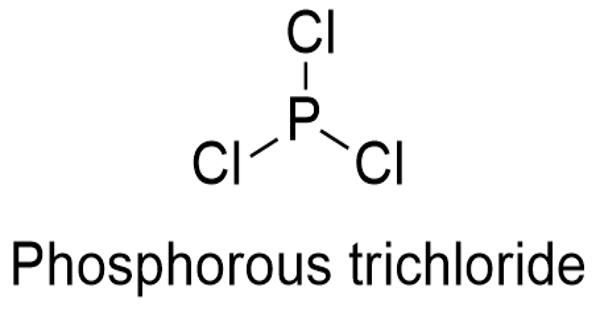
Phosphorus trichloride is an essential raw material in the production of oxyphosphorus compounds for commercial use. It is an inorganic compound with the chemical formula PCl3. It is produced by the reaction of yellow phosphorus and chloride. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds.
It is a toxic and reacts violently with water to release hydrogen chloride.
Properties
- Molecular Weight: 137.33
- Appearance: Clear liquid
- Melting Point: 118 °C
- Boiling Point: 76 °C
- Density: 1.574 g/cm3
- Solubility in H2O: Reacts violently
- Exact Mass: 135.88032 g/mol
Preparation
World production exceeds one-third of a million tonnes. Phosphorus trichloride is prepared industrially by the reaction of chlorine with white phosphorus, using phosphorus trichloride as the solvent. It is manufactured by burning molten white phosphorus in dry chlorine. In this continous process PCl3 is removed as it is formed in order to avoid the formation of PCl5).
P4 + 6 Cl2 → 4 PCl3
Phosphorus trichloride on further chlorination forms Phosphorus pentachloride. The chemical reaction is given below.
PCl3 + Cl2 → PCl5
Industrial production of phosphorus trichloride is controlled under the Chemical Weapons Convention, where it is listed in schedule 3. In the laboratory it may be more convenient to use red phosphorus. An explosion occurs when phosphorus trichloride is brought in contact with nitric or nitrous acid.
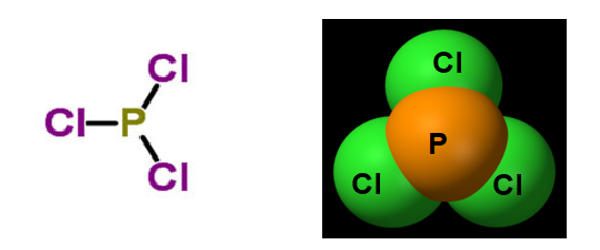
Structure
It has a trigonal pyramidal shape. Its 31P NMR spectrum exhibits a singlet around +220 ppm with reference to a phosphoric acid standard.
Effective control of the phosphorus-to-chlorine ratio is essential if product yield is to be maximized. Proper control also ensures that an appropriate amount of heat is generated, while maintaining the stoichiometry for the trichloride’s production. Enough heat will be generated by the reaction itself to distill the product as it is formed.
Uses
It has many commercial importances. These commercial uses are highly varied and can include oil and polymer additives, phosphate esters, pest control compounds, special lubricants and fire-resistant materials.
- The Largest use for phosphorus trichloride is for making phosphorus oxychloride by oxidizing it with oxygen.
- PCl3 is important indirectly as a precursor to PCl5, POCl3 and PSCl3, which are used in many applications, including herbicides, insecticides, plasticisers, oil additives, and flame retardants.
- It is a colourless transparent motile liquid. It is the most important phosphorus halogen.
- PCl3 is the precursor to triphenylphosphine for the Wittig reaction, and phosphite esters which may be used as industrial intermediates, or used in the Horner-Wadsworth-Emmons reaction, both important methods for making alkenes.
- It is used as chlorinating agent for converting alkyl alcohols to alkyl chlorides and organic acids to organic acid chlorides and also as a catalyst.
- It can be used to make trioctylphosphine oxide (TOPO), used as an extraction agent, although TOPO is usually made via the corresponding phosphine.
- PCl3 is also used directly as a reagent in organic synthesis. It is used to convert primary and secondary alcohols into alkyl chlorides, or carboxylic acids into acyl chlorides, although thionyl chloride generally gives better yields than PCl3.
Health Hazards
A phosphorus trichloride produces acute systemic effects by absorption through the skin into the bloodstream.
Information Source: