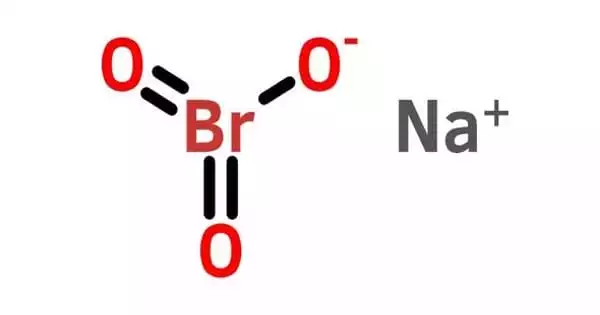
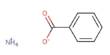
The sodium salt of bromic acid is sodium bromate, an inorganic molecule having the chemical formula NaBrO3. It is a powerful oxidant. It is a strong oxidizing agent that can explode if exposed to fire or heat for an extended period of time. It is an effective brominating agent for aromatic compounds with deactivating substituents.
Properties
Sodium bromate has the appearance of a white crystalline solid. It is an inorganic sodium salt with the counterion bromate. It functions as an oxidizing agent as well as a nephrotoxin. It is a bromate salt as well as an inorganic sodium salt. It may explode if exposed to heat or fire for an extended period of time.
- Molar Mass/Molecular Weight: 150.891 g/mol
- Color and Appearance: Colorless to white crystals, dry powder
- Odor: Odorless
- Melting Point: 381 °C, 717.8 °F (decomposes with evolution of oxygen)
- Boiling Point: 1390 °C, 2530 °F
- Density: 3.34 g cm-3
- State of matter at room temperature: Solid
- Solubility: Soluble in ammonia and insoluble in ethanol
Composition and Synthesis
When bromine reacts with a concentrated, heated solution of sodium carbonate, it produces sodium bromate, sodium bromide, and carbon dioxide as the products.
3Br2 + 3Na2CO3 → 5NaBr + NaBrO3 + 3CO2
Sodium bromate is formed through electrolytic oxidation of sodium bromide in which stainless steel plate works as the cathode and mixed metal oxide coated titanium plate acts as the anode.
NaBr + 3H2O → NaBrO3 + 3H2

Production
Sodium bromate is created by combining sodium hydroxide and bromic acid. It can also be produced through the electrolysis of a sodium bromide solution. It is possible to make it by dissolving bromine in a heated sodium hydroxide solution. This also results in the production of sodium bromide.
Bromine is converted into sodium bromate by passing it through a sodium carbonate solution. It can also be generated electrolytically by oxidizing sodium bromide. It can also be produced by oxidizing bromine with chlorine to sodium hydroxide at 80 °C.
3 Br2 + 3 Na2CO3 = 5 NaBr + NaBrO3 + 3 CO2
Uses
When combined with sodium bromide, sodium bromate is mostly employed in continuous or batch dyeing operations employing sulfur or vat dyes, as well as as a hair-permagent, chemical agent, or gold solvent in gold mines. It is used to extract gold from ore in conjunction with sodium bromide. It is also employed as an analytical reagent, a boiler cleaner, and a component in hair-waving formulas.
Human health issues
Bromate in drinking water is undesirable since it is a possible carcinogen in humans. It is known to produce acute poisoning through eating, inhalation, and skin and ocular contact. It causes severe skin irritation, eye damage, and respiratory tract inflammation. Its presence in Coca-Dasani Cola’s bottled water prompted a recall in the United Kingdom.