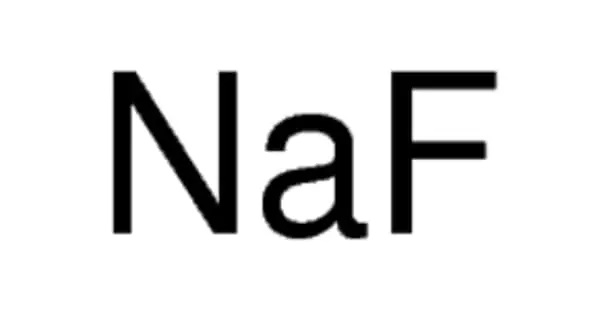The inorganic compound sodium fluoride (NaF) has the formula NaF. It is a colorless crystalline solid, white powder, or a solid dissolved in a liquid. It is used in trace amounts in drinking water fluoridation, toothpaste, metallurgy, and as a flux, as well as pesticides and rat poison. It dissolves in water. It is not flammable. It occurs in nature as the rare mineral villiaumite, in very small quantities.
It is a colorless or white solid that dissolves easily in water. It is a common source of fluoride in pharmaceutical production and is used to prevent dental cavities. With over one million prescriptions in 2017, it was the 247th most commonly prescribed medication in the United States.
Properties
Sodium fluoride is found as an odorless, crystalline solid that is white to greenish in color, depending on its purity. Its density is 2.56 g/mL, its melting point is 993 °C, and its boiling point is 1,704 °C. It is a hygroscopic solid (absorbs moisture from the air).
- Melting point: 993 °C (lit.)
- Boiling point: 1700 °C
- Density: 1.02 g/mL at 20 °C
- Vapor pressure 1.4 mm Hg ( 0 °C)
- Refractive index 1.336
- Flash point: 1704°C
- Storage temp.: 2-8°C
- Solubility H2O: 0.5 M at 20 °C, clear, colorless
- Form: powder
- Color: White to off-white
- Specific Gravity: 2.558
- Odor: Odorless

Structure
Sodium fluoride has the chemical formula NaF and a molar mass of 41.99 g/mol. It is a straightforward ionic compound composed of sodium (Na+) cation and fluoride (F-) anion. The solid salt exists in the form of cubic crystals, which are similar to the crystal structure of sodium chloride (NaCl).
Production
The main source of sodium fluoride is industrial production. It is usually made by neutralizing hydrofluoric acid with a base such as sodium carbonate (soda ash, Na2CO3), sodium hydroxide (caustic soda, NaOH), or sodium bicarbonate (NaHCO3).
HF + NaOH → NaF + H2O
NaF is made by neutralizing hydrofluoric acid or hexafluorosilicic acid (H2SiF6), both byproducts of the reaction of fluorapatite [Ca5(PO4)3F] from phosphate rock during superphosphate fertilizer production. Sodium hydroxide and sodium carbonate are examples of neutralizing agents.
Sodium fluoride precipitates as the bifluoride salt sodium bifluoride from HF solutions (NaHF2). Heating the latter produces HF and NaF.
HF + NaF ⇌ NaHF2
In a 1986 report, the annual worldwide consumption of NaF was estimated to be several million tonnes.
Uses
Sodium fluoride is sold in tablets for cavity prevention.
- Dental caries – Fluoride salts are frequently added to municipal drinking water (and, in some countries, to certain food products) to maintain dental health. Fluoride strengthens teeth by promoting the formation of fluorapatite, a naturally occurring component of tooth enamel.
- Osteoporosis – Fluoride supplementation for the treatment of postmenopausal osteoporosis has been extensively researched. This supplement appears to be ineffective; while sodium fluoride increases bone density, it does not reduce the risk of fractures.
- Medical imaging – Fluorine-18-labelled sodium fluoride (USP, sodium fluoride F18) is one of the oldest tracers used in positron emission tomography (PET), having been in use since the 1960s.
- Chemistry – Sodium fluoride has a wide range of specialty chemical applications in synthesis and extractive metallurgy. It reacts with electrophilic chlorides such as acyl chlorides, sulfur chlorides, and phosphorus chloride.
Other uses
- Sodium fluoride is used as a cleaning agent (e.g., as a “laundry sour”).
- It can be used in a nuclear molten salt reactor.
- Over a century ago, it was used as a stomach poison for plant-feeding insects. Inorganic fluorides such as fluorosilicates and sodium fluoride complex magnesium ions as magnesium fluorophosphate.
Safety
Sodium fluoride is a poisonous and corrosive substance. It can cause abdominal pain, vomiting, diarrhea, convulsions, collapse, and even death if swallowed. Inhalation can cause severe respiratory tract irritation. Skin or eye contact with solid NaF can cause severe irritation or burns, as well as severe injury or death.
Fluorides, particularly sodium fluoride aqueous solutions, are rapidly and extensively absorbed by the human body. Although fluoride is safe for dental health at low concentrations, consuming large amounts of soluble fluoride salts on a regular basis is dangerous.
Chronic overabsorption can result in bone hardening, ligament calcification, and plaque buildup on teeth. Fluoride can irritate or corrode eyes, skin, and nasal membranes.