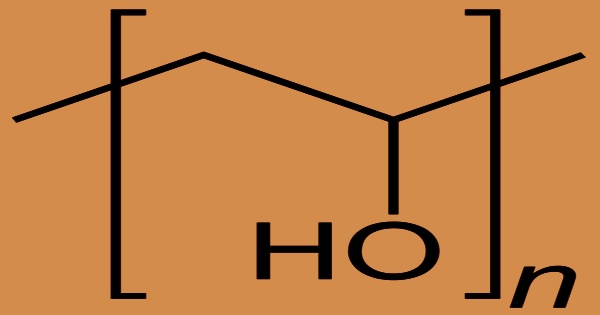
The water-soluble synthetic polymer is polyvinyl alcohol (PVA), also known as PVOH or PVAL, which has the idealized formula (CH2CH(OH)n. It works well for film formation, emulsification, and adhesion. It has no odor, is non-toxic, and is grease, oil, and solvent resistant. Mold can grow in pure aqueous solutions because they are neutral or slightly acidic. It’s ductile but strong, flexible, and acts as excellent oxygen and odor barrier. Polyvinyl alcohol is used in papermaking, textile warp sizing, PVAc adhesive formulations, and a variety of coatings as a thickener and emulsion stabilizer. It is odorless and colorless, and it is commonly supplied as beads or as water solutions.
Polyvinyl alcohol is unusual among polymers (chemical compounds made up of large, multiple-unit molecules) in that it is not made up of single-unit precursor molecules known as monomers during polymerization reactions. It is formed by the hydrolysis of polyvinyl acetate rather than polymerization of monomers; the molecular backbone contains -CH2-CH(OH)-. The hydrolysis, or “alcoholysis,” reaction that follows eliminates the acetate groups from the PVAc molecules without disturbing their long-chain structure.
Typically, base-catalyzed transesterification with ethanol is used to convert polyvinyl esters:
(CH2CH(OAc))n + C2H5OH → (CH2CH(OH))n + C2H5OAc
The degree of transesterification has an effect on the polymer’s properties. This substance has a specific gravity of 1.25 to 1.35 and a melting point of 212 to 267 degrees Celsius. In hot water and hot dimethyl sulfoxide, it dissolves. Animal tests indicate that subcutaneous, intramuscular, and intravenous injections of polyvinyl alcohol cause no major toxicity when given without stimulation. While other vinyl polymers are made by polymerizing the corresponding monomer, PVA is made by extracting the acetate groups from polyvinyl acetate by partial or full hydrolysis. Sizing is a manufacturing mechanism applied to rovings and yarns to ensure lubricity and binding action in order to protect the fibers and assist in handling.
Polyvinyl alcohol resin materials are white solids with sub-floc, granular, and powder appearances; they are non-toxic, tasteless, and non-polluting, and they are soluble in water at 80-90 °C. In 2006, over one million metric tons of polyvinyl alcohol were consumed worldwide. Kuraray (Japan, Europe, and the United States) and Sekisui Specialty Chemicals (US) are major producers, while mainland China has established a number of very large production facilities in the last decade and now accounts for 45 percent of global output. Its aqueous solution has strong adhesion and film-forming properties, and it can withstand most organic solvents such as oils, lubricants, and hydrocarbons. It also has long-chain polyol esterification, etherification, and acetalization properties, among other chemical properties.
Vinyl acetate monomer is the primary raw material used to make PVA. Polymerization of vinyl acetate is used to create the monomer. PVA is a crystallinity-exhibiting atactic substance. In terms of microstructure, it’s mostly made up of 1,3-diol linkages (-CH2-CH(OH)-CH2-CH(OH)-), with a small percentage of 1,2-diols (-CH2-CH(OH)-CH(OH)-CH2-) thrown in for good measure, depending on the vinyl ester precursor’s polymerization conditions. Polyvinyl alcohol is a synthetic resin made by polymerizing vinyl acetate and then hydrolyzing the ester partially in the presence of an alkaline catalyst. The degree of polymerisation and hydrolysis determine the physical characteristics of the product.
PVA is made from polyvinyl acetate after it has been hydrolyzed. Because it is difficult to obtain in the quantities and purity required for polymerization, the repeating unit of vinyl alcohol is not used as the starting material. Using alkalis or mineral acids as catalysts, the hydrolysis proceeds quickly in methanol, ethanol, or a mixture of alcohol and methyl acetate. The 4 percent aqueous solution of Polyvinyl alcohol (PVA) can be made in one of two ways. The first uses a traditional heating method, while the second uses microwave technology. The latter is less time-consuming and easier to conduct. The 4 percent PVA solution can last between 6 and 8 weeks once made. It’s best to keep this solution refrigerated because it encourages bacteria growth.
Oil, grease, and solvents are all resistant to PVA. It has excellent tensile strength and flexibility, as well as excellent oxygen and odor barrier properties. Polyvinyl alcohol (PVA) is a tough, whitish polymer that can be formed into strong films, tubes, and fibers that are hydrocarbon-resistant. Despite being one of the few water-soluble polymers, polyvinyl alcohol can be rendered insoluble in water by drawing or using cross-linking agents. PVA is added to bags of oil-based or dry fishing bait that are then attached to the hook. Because PVA is soluble in water, it breaks down when the bag lands on the water’s surface, leaving the hook bait surrounded by pellets and ground bait. Although the PVA causes the plastic to dissolve in water, it attracts fish to the hook bait.
Polyvinyl alcohol is also used in the manufacture of surface coatings, artificial sponges, cosmetics, and other products, as well as as a pharmaceutic aid and ophthalmic lubricant. Because of its biocompatibility, low tendency for protein adhesion, and low toxicity, PVA is used in a variety of medical applications. Cartilage replacements, contact lenses, and eye drops are all examples of specific applications. It’s also worth noting that polyvinyl alcohol is widely used for coating medicinal tablets in another rapidly growing market, the pharmaceutical industry. Its various chemical properties have demonstrated the value of using it in health-related applications, particularly pharmaceutics.
Polyvinyl alcohol is generally thought to be a non-toxic substance. It is non-irritating to the skin and eyes at concentrations up to 10%; cosmetics use concentrations as low as 7%. When kept in a tightly sealed container in a cool, dry location, it remains stable. In corrosion-resistant sealed containers, aqueous solutions are stable. If the solution needs to be stored for a long time, preservatives can be added. Because PVA is widely used, researchers are interested in learning more about its toxicity and biodegradation. Fish are unaffected by solutions containing up to 5% PVA, and it biodegrades slowly. Although they are soluble in both hot and cold water, those coded 20-105 require alcohol-water mixtures; Petroleum solvents are insoluble.
Information Sources: