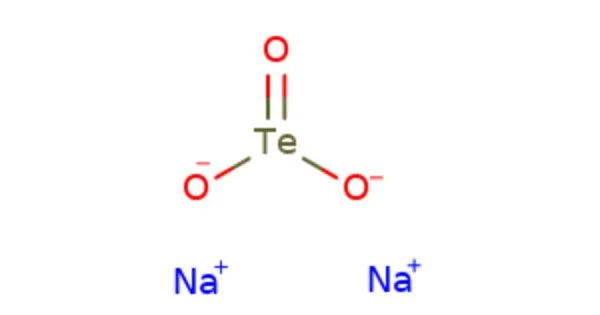
Sodium tellurite is an inorganic compound with the chemical formula Na2TeO3. It is a white crystalline powder that is soluble in water. It is a source of the tellurium ion TeO32-, which is often used in the production of glass, ceramics, and inorganic pigments. It is also used in microbiology as a selective agent to promote the growth of certain types of bacteria, particularly members of the Enterobacteriaceae family.
In this application, it is added to culture media to inhibit the growth of other microorganisms and allow for the isolation and identification of specific bacterial species. Sodium tellurite is also used in analytical chemistry to detect the presence of gold and in electroplating to deposit tellurium coatings on metal surfaces. It is important to note that sodium tellurite is toxic and can be hazardous if not handled properly.
It is an inorganic tellurium compound with formula Na2TeO3. It is a water-soluble white solid and a weak reducing agent. Sodium tellurite is an intermediate in the extraction of the element, tellurium; it is a product obtained from anode slimes and is a precursor to tellurium.
Properties
- Chemical formula: Na2TeO3
- Molar mass: 221.57774 g/mol
- Appearance: white crystals, powder
- Density: 6.245 g/cm3
- Melting point: 710 °C (1,310 °F; 983 K)
- Solubility in water: soluble (greater than or equal to 100 mg/mL at 68°F)
- Crystal structure: rhombic

Preparation
The main source of tellurium is from copper anode slimes, which contain precious metals as well as various tellurides. These slimes are roasted with sodium carbonate and oxygen to produce sodium tellurite.
Ag2Te + Na2CO3 + O2 → 2Ag + Na2TeO3 + CO2 (400–500 °C)
This is a reaction with silver telluride. The telluride is oxidized to tellurite and the silver(I) is reduced to silver.
Applications
The corrosion resistance of electroplated nickel layers is improved by sodium tellurite. Sodium tellurite solutions are used to coat iron, steel, aluminum, and copper with black or blue-black coatings. In microbiology, sodium tellurite can be added to the growth medium to isolate bacteria that have a built-in physiological resistance to its toxicity.
Sodium tellurite is a common precursor to other tellurium compounds and is used in the production of glass, ceramics, and pigments. However, sodium tellurite is toxic and exposure to it can cause serious health problems, including respiratory problems, skin irritation, and eye damage. Therefore, it should be handled with care and only by trained professionals.