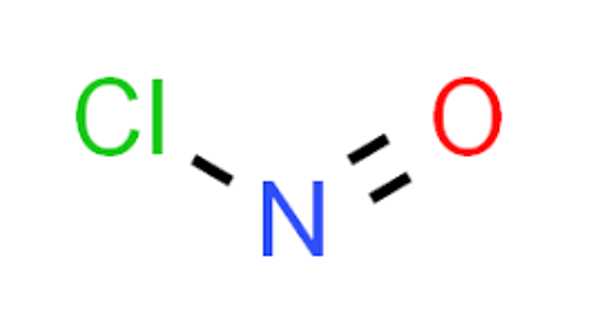
The chemical compound with the formula NOCl is nitrosyl chloride. It is a yellow gas that is most commonly encountered as a byproduct of the decomposition of aqua regia, a mixture of hydrochloric and nitric acid. It is a powerful electrophile and oxidizer. Tilden’s reagent is another name for it.
Properties
Nitrosyl Chloride is an orange-red gas or a dark red liquid with an unpleasant odor. It is used as a catalyst, an intermediate, a flour bleaching agent, and in synthetic detergents. It has the appearance of a yellow to yellowish-red gas. At -5.5°C, it liquefies. It is extremely toxic when inhaled.
- Appearance: Yellow gas
- Density: 2.872 mg mL-1
- Melting point: −59.4 °C (−74.9 °F; 213.8 K)
- Boiling point: −5.55 °C (22.01 °F; 267.60 K)
Structure and synthesis
The molecule is bent. A double bond exists between N and O (distance = 1.16 Å) and a single bond between N and Cl (distance = 1.96 Å). The O–N–Cl angle is 113°.
Noncombustible, but hastens the combustion of combustible materials. Water decomposes it to form corrosive hydrochloric acid. In general, vapors are heavier than air. Long-term exposure to fire or heat can cause containers to explode violently.
Production
A pure sample of nitrosyl chloride was made by either reacting phosphorus trichloride with concentrated nitric acid or reacting phosphorus trichloride with sodium nitrate in the presence of water. I.R. spectral data and elemental analysis were used to characterize the nitrosyl chloride gas.
Since nitrosyl chloride is chemically simple and thermally stable, it can be produced in many ways.
Combining nitrosylsulfuric acid and HCl affords the compound. This method is used industrially.
HCl + NOHSO4 → H2SO4 + NOCl
A more convenient laboratory method involves the (reversible) dehydration of nitrous acid by HCl.
HNO2 + HCl → H2O + NOCl
Michael Faraday prepared nitrosyl chloride by reacting palladium with aqua regia:
Pd + HNO3 + 3 HCl → PdCl2 + 2 H2O + NOCl
NOCl forms by the direct combination of chlorine and nitric oxide; This reaction reverses above 100 °C.
Cl2 + 2 NO → 2NOCl
Another method of producing nitrosyl chloride is by direct union of the elements at 400 °C, although there is some regression as above.
N2 + O2 + Cl2 → 2 NOCl ⇌ 2 NO + Cl2
Industrial applications
Cyclohexanone oxime hydrochloride is formed when NOCl and cyclohexane react photochemically. This method takes advantage of NOCl’s proclivity for photodissociation into NO and Cl radicals. Caprolactam, a precursor to Nylon-6, is formed from the oxide.
Health Risks
The inhalation of Nitrosyl Chloride can irritate the lungs, resulting in coughing and/or shortness of breath. Higher levels of exposure can result in a buildup of fluid in the lungs (pulmonary edema), a medical emergency characterized by severe shortness of breath.